Introduction
Prostate cancer is the second most common cancer among men, after skin cancer1 and the fifth-leading cause of death, accounting for 6.6% of all cancer deaths, worldwide. Mortality is higher in developing countries, doing special mention to the black populations.1 Conversely, the lowest mortality rate belongs to the Asian population.2
In Spain, prostate cancer is the most frequently diagnosed cancer in men and the second one when both sexes are considered. In 2010 there was an incidence of 27 853 new cases of prostate cancer, representing 12.9% of all diagnosed tumors. In men, prostate cancer is the third leading cause of death, which corresponds to 8.6% of the total deaths caused by cancer.3
Age, family history and race are the only three risk factors which have shown to be related to the likelihood of developing prostate cancer.4
The management of prostate cancer has been based on local treatments (radical prostatectomy and radiotherapy) and androgen deprivation that managed to control the disease temporarily. However, over time, patients begin to show signs of disease progression, with increased Prostate Specific Antigen. This state is known as castration-resistant prostate cancer (CRPC), where the prognosis is worse than first stage prostate cancer, and the median overall survival (OS) ranges between 9 to 30 months.5 Although at the time of diagnosis, between 3% and 8% of patients have bone symptoms, of those, up to 90% will develop bone metastasis in the next 15 years (mCRPC).5 In this stage of the disease appears a set of complications known as a symptomatic skeletal event (SSE). SSE includes pathologic fractures, spinal cord compression, bone surgery and radiotherapy in the bones.6 On average, a patient with mCRPC will have a SSE every 3-6 months (IPT radium-223 dichloride, 2015). SSEs are associated with impaired mobility, reduced quality of life and increased health care costs.7
Currently, there are effective treatments to reduce pain and delay the SSE, such as denosumab and zoledronic acid, but these treatments do not offer a benefit on patient survival.7 Conversely, available active treatments have shown survival benefits in mCRPC without proving benefit on SSE. These active treatments include chemotherapy treatments as docetaxel8 and cabazitaxel9, and hormone therapies as abiraterone acetate (hereinafter, abiraterone)10 and enzalutamide.11 Recently radium-223 dichloride (hereinafter, radium-223), a bone-targeted alpha therapy, has shown impact on OS and delay of disease progression and SSEs.6
Therefore, during the last few years, the therapeutic options for the treatment of mCRPC has been expanded. Despite this fact, we cannot forget that health systems have limited resources, and therefore, the efficiency and cost-effectiveness of the new treatments have become a requirement in most of the countries.
Therefore, the aim of this paper is to assess the cost-effectiveness of radium-223 compared with BSC in a population of patients with mCRPC who were previously treated with docetaxel in Spain.
Design and Methods
To meet the objectives previously described, a cost-effectiveness analysis has been carried out estimating the costs and the health outcomes achieved by patients treated with the new intervention, radium-223 plus BSC versus BSC alone.
The population included in the assessment was those mCRPC patients without previous treatment with docetaxel, neither other chemotherapy. A health system perspective was adopted to conduct the economic evaluation.
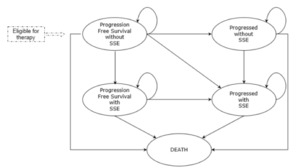
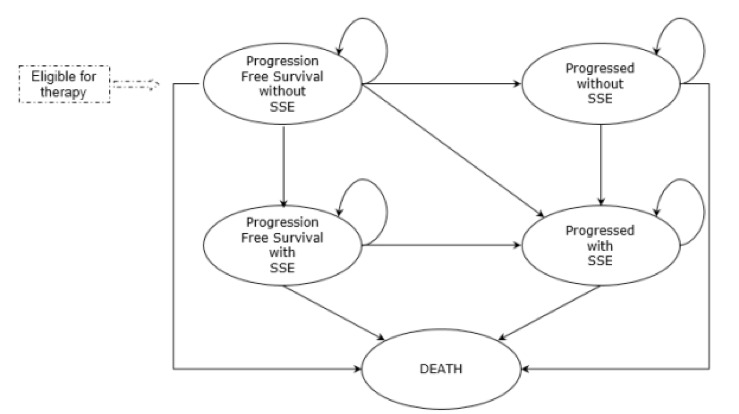
A pharmacoeconomic Markov model has been developed in Microsoft Excel® to ensure transparency and flexibility. The model includes five mutually exclusive health states that allows simulate the evolution of mCRPC (Figure 1). All the patients initiate the simulation in the health state called “progression-free survival without SSE”. At the end of each cycle patients can move to one of the other four health states: “progression without SSE”, “progression-free survival with SSE”, “progression with SSE” and “death” or remain in their initial health state. The model used a cycle length of 1 week to allow an adequate simulation of the rapid disease progression.
The time horizon for the assessment was 10 years. It was deemed enough due to the fact that live expectancy in this population is limited and therefore 10 years are considered a lifetime horizon allowing to capture all costs and health benefits of both comparators.
Inputs Used to Populate the Model
Effectiveness
The effectiveness of the alternative treatments has been based on one randomized clinical trial, using the information on the target pre-chemotherapy population.6
The ALSYMPCA trial is a phase III randomized, double-blind, multi-country trial to assess the efficacy and safety of radium-223 plus BSC for the treatment of mCRPC patients versus placebo plus BSC.
In brief, in the ALSYMPCA trial a total of 921 patients were recruited, of those, 526 received previous treatment with docetaxel, while the 395 remaining patients had not received previous treatment with chemotherapy (no previous docetaxel group). There were no significant differences between these two groups regarding basal characteristics of patients beyond the previous use of docetaxel. The patients were randomized in a ratio 2:1, to radium-223 (55 KBq/Kg every 28 days for a maximum of 6 cycles) or placebo, respectively. For the present evaluation, only the data of the no previous docetaxel subgroup of patients has been used.
The BSC was administered to both treatment arms and defined as the routine treatment provided at each clinical centre, including: external radiotherapy, corticosteroids, antiandrogens, ketoconazole, diethyl estilbestrol, or estramusina.6
The primary and the secondary objectives of the ALSYMPCA trial were achieved: an OS with reduced risk of death by 30% and time to first SSE, time to increase alkaline phosphatase, time to prostate-specific antigen increase, quality of life, and safety were favorable to patients randomized to radium-223. Additional information can be seen elsewhere.6
The main variables that determine treatment efficacy, time to death, time to disease progression and time to SSE were assessed through survival analyses. To extrapolate the treatment efficacy observed during the study follow-up to 10 years of the time horizon used in the model, parametric survival curves were used. The estimation was carried out from the individual data of the subgroup of patients without previous docetaxel treatment, included in the ALSYMPCA study. Several survival models were tested: exponential, log-normal, log-logistic and Weibull. The log-normal function had been selected for the economic evaluation for being the one that best fits to the trial data, based on visual and statistical criteria, Akaike Information Criterion (AIC). The clinical inputs used to populate the model are shown in Table 1.
Utility Scores / Quality of Life
In cost-utility analysis QALYs are calculated as a standard method to quantify the quality of life in each health state. QALYs are calculated by multiplying the utility value of the health state (a measure of quality of life reported by the patient in a questionnaire referred to his health status) for the time spent in each health state. The quality of life measures were also obtained from ALSYMPCA trial. Disease progression and SSE have an impact on quality of life.
In the ALSYMPCA trial, quality of life of patients were obtained with the European Quality of Life-5 Dimensions (EQ-5D) questionnaire collected at different time points during the trial: in the basal visit, in two visits along the treatment period (weeks 16 and 24) and then during the follow-up visits. Utility observations that were missing or where utility date was missing were excluded. By the end of the study, the average number of utility score measurements was 3.8 for radium-223 patients and 3.2 for placebo patients (3178 utility scores over 882 patients). During the treatment period the utility values for patients treated with radium-223 and BSC were different, but once the disease advance it was assumed the same utility values for both treatments. The mean observed values by treatment and progression state are shown in Table 2.
Resource Use and Costs
The use of health resources has also been collected in the ALSYMPCA trial. It includes hospital days, day care use and physician visits. Other health resources, distinguishing between pre-progression and post-progression health states but not according to the treatment, are valued as described by expert opinion.
Unit costs were retrieved from Spanish databases and published sources.12,13
The drug prices were valued at ex-factory price notified applying the 7.5% deduction of established by Royal Decree Law 8/2010 of 20 May when necessary.
Other unit costs included in the model were: the costs of administration (in the case of intravenous infusion (IV) of radium-223); cost of drugs to treat adverse events; cost of procedures for monitoring patients; cost of the treatments of SSE; cost of the “end of life” treatments; and costs of other resources such as the day of hospitalization, day-care unit and physician visits. All data referring to resource use and unit costs are collected in Table 3.
Analysis
The base case analysis was conducted from a National Health System perspective and used QALYs as health outcome measure, and the following results for each comparator are displayed, total discounted cost, total discounted QALYs, total life years, incremental costs, incremental QALYs and incremental life-years. Finally, incremental cost-effectiveness ratio (ICER) was estimated as follows:
ICER =costRadium−223−costBSCQALYRadium−223−QALYBSC
Additionally, cost breakdown was also presented.
Uncertainty
Because of the parameters uncertainty inherent in any model, it is recommended to perform a sensitivity analysis to test the robustness of the results, that is, to what extent the variation of the main variables can alter the final results. One-way and probabilistic sensitivity analyses were conducted to assess the uncertainty associated with parameter estimations and the robustness of the base case results.
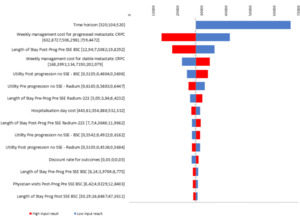
A tornado diagram presenting the 10 parameters which have a greater impact in the results of the economic evaluation was developed.
The probabilistic sensitivity analysis was performed by means of 1000 cohort simulations, and results were displayed by a cost-effectiveness acceptability curve. Gamma and beta distributions were used to run simulations; gamma distribution for resource use and unit costs; and beta distribution for rates, probabilities and utility scores.
Future costs and health outcomes were discounted at an annual rate of 3% in the base case analysis. In one-way sensitivity analysis, rates of 0% and 5% were applied as recommended in Spanish guidelines.14
Results
Base Case
Patients treated with radium-223 achieve a mean of 1.12 QALYs, improving in 0.35 the results achieved by patients treated with BSC. The total cost per patient treated with radium-223 was €65 067; this involves an increment of €9631 cost for a patient treated with BSC. Thus, the ICER of radium-223 versus BSC for the treatment of mCRPC without previous chemotherapy treatment was €27 606 per QALY gained (Table 4).
The incremental benefit in LYs gained achieved by radium-223 was 0.40.
A detailed analysis of cost breakdown (Table 5), shows that radium-223 increased the drug costs in €27 195 but allows to save €14 643 in the management of the patient, plus €3255 in avoided hospitalizations. Other cost groups were similar between treatments.
One-way Sensitivity Analysis
The survival curves used to model progression and OS show a great impact on the results (Table 6).
The one-way sensitivity analysis (OWSA) showed that time horizon was the most influential parameter, the ICER of radium-223 raised with the decrease of the time horizon. It seems evident because in shorter time horizons it is not allowed to retrieve the whole clinical benefit, but drug costs were generated. Other parameters with a remarkable impact on results were weekly management cost and length of stay in postprogressed patients; the smaller they were the less efficient the radium-223 was, since less value has the delay in progression achieved with radium-223 (Figure 2).
Probabilistic Sensitivity Analysis
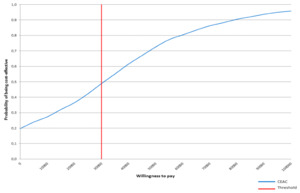
In the cost-effectiveness acceptability curve (CEAC; Figure 3) we can see that the probability of being costeffective for radium-223 was 48% with a willingness-to-pay of €30 000 per QALY, suggesting there was a high uncertainty regarding which alternative compared was the best option for mCRPC patients.
Discussion
Our National Health System is a system of limited resources, and each year must attend a multitude of applications for approval of new health interventions. In the pursuit of sustainability of the system, the government must assess what additional costs are associated with new alternatives and if the benefits derived justify such investments. This leads to a growing need for economic evaluations. That is why the growing need for economic evaluations that facilitate decision-making regarding new drugs and medical devices.
In this analysis of cost-effectiveness analysis, we evaluated radium-233 versus BSC among patients with mCRPC without previous use of docetaxel has been evaluated. Our analysis, which has been conducted from a health system perspective, indicates that the ICER is below €30 000 per QALY gained.
No previous economic evaluations of radium-233 in Spain were found. However, they exist in other settings. In the article written by Norum et al., concluded that the use of radium-223 was not cost-effective or at least there were not enough data to conclude that it was.15 Renzulli et al., stated in his article the experience of a multidisciplinary approach in the treatment of mCRPC, and concludes that radium-223 has demonstrated overall survival and delayed onset of SSE regardless of whether it was administered or not previously to docetaxel.16 Gaultney et al., developed a Markov model for analysis of cost-effectiveness analysis with radium-223 and several comparators in the Netherlands.17 The results conclude that, given abiraterone, radium-223 is cost-effective, that is, radium-223 is more efficient but also more expensive than abiraterone, although below the threshold of willingness-to-pay. Finally, Henricks et al., work also developed a Markov model where the delay in the onset of SSE and the costs associated with hospitalization by radium-223 make it to be cost-effective compared to BSC.18
The robustness of our base case results were tested in the sensitivity analysis. In the one-way sensitivity analysis, the time horizon considered was the parameter with a higher impact on the results was the time horizon considered: the shorter it was the less efficient radium-223 resulted. The higher uncertainty is related with the type of survival curve selected to extrapolate trial results on a longer time horizon, but in the base case analysis the Akaike Information Criterion was used to determine which parametric curve better fits the Kaplan-Meier data.
In the probabilistic sensitivity analysis and considering a willingness-to-pay of €30 000 per QALY, there is a probability of 48% that radium-233 is cost-effective against its comparator. This probability increases to 66% and with a willingness to pay of €45 000 per QALY. For Spain, the threshold of efficiency, from which, a new health intervention is considered cost-effective, the literature so far provided us with the limit of efficiency in €30 000 - €45 000 per QALY. Referring to our country, the literature has provided the efficiency threshold of €20 000 - €45 000 per QALY, when establishing a threshold of efficiency from which a new health intervention is considered cost-effective.19–21
There are a number of limitations to be noted. The first one was the use of same utility scores for both arms once the disease advanced. Utility scores included in the model were collected from patients included in the ALSYMPCA trial.6 This allowed the use of different utility scores in both arms during the active treatment period, but not longer. However, after the progression of the disease, patients discontinued their treatments, so the quality of life should be similar regardless of their initial treatments. Another limitation was the fact that the use of healthcare resources was also collected from the ALSYMPCA trial. Therefore, the typical standard of care differs from the care provided during a trial, which may have biased the results. Nevertheless, utility scores are considered a better proxy than to define healthcare resource use by an Advisory Board. The third limitation was the use of BSC instead of another active treatment. Active treatment use involves the development of an indirect meta-analysis comparison. In Spain, abiraterone should be a possible alternative therapy for mCRPC patients without previous docetaxel treatment. However, populations included in radium-223 and abiraterone trials were not considered similar enough to perform indirect comparisons.6,22
In conclusion, from the Spanish National Health System perspective and based on the results of the present analysis, radium-223 could be a suitable option of health resource utilization for end-of-life mCRPC patients without previous docetaxel treatment, subject to a moderate level of uncertainty.
Conflict of Interest Declaration
CMA, MGV, and ETM are currently employees of Bayer Hispania. MRC and DCV are employees of IQVIA.