BACKGROUND
Although the etiologies of the chronic retinal vascular diseases neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and retinal vein occlusion (RVO) differ, the vascular endothelial growth factor (VEGF) pathway is a pivotal component of the pathophysiology underlying all 3 conditions.1 Another common feature of these 3 disease states is that all can result in severe vision loss if left untreated.2
Previous research has linked having poor vision to an increased risk of falls and associated injuries, including fractures.3–6 Self-reported vision impairment also has been linked to an increased fear of falling, which can lead to activity limitation and associated declines in quality of life.4 Recently, psychological distress was reported in 26% of the adults who have difficulty seeing even when wearing glasses or contact lenses.7 In fact, patients with age-related macular degeneration (AMD) are at particularly high risk of depression compared with patients with other eye diseases.8 For community-dwelling older people, those with macular degeneration have twice the incidence of depression as those without, driven by both functional decline and loss of leisure activities.9
Vision loss has also been associated with disabilities in activities of daily living (ADLs) and instrumental activities of daily living (iADLs).10 Visually impaired individuals 60 years of age and over were recently shown to have significantly lower mean iADL scores compared with those without vision impairment, with iADL score being significantly correlated with near visual acuity.11 The relationship between retinal diseases and patient’s functional status relative to the Medicare population without vision-threatening retinal diseases (VTRDs) is not well described. Thus, this retrospective, real-world study sought to assess differences in perceived visual function, ADLs, iADLs, falls/fractures, and depression/anxiety between patients with nAMD, DME, or RVO and those without these or other common VTRDs, using data derived from the Medicare Current Beneficiary Survey (MCBS) linked with Medicare Fee-for-Service (FFS) claims.
METHODS
Study Design
This study analyzed pooled cross-sectional MCBS data linked with Medicare FFS claims from 2006 to 2013 and 2015 to 2018. The year 2014 was not included since 2014 data were not released by the Centers for Medicare & Medicaid Services.12 The MCBS is a continuous in-person longitudinal survey that collects individual-level data on beneficiaries’ largely self-reported sociodemographics, health status and functioning, access to care, health insurance coverage and expenses, financial resources, and family support. By design, the MCBS cross-sectional data are representative of the population of all Medicare beneficiaries for any given survey year.13 This study was exempt from Institutional Review Board approval due to de-identified data.
Study Population
The study population consisted of community-dwelling (ie, not living in a nursing home or other facility) adult (≥18 years) Medicare beneficiaries who had full-year Medicare Parts A and B enrollment in the same year of administration of the MCBS survey. The disease cohorts included beneficiaries who were diagnosed with nAMD, DME, or RVO. To increase diagnostic specificity, beneficiaries with at least 1 inpatient diagnosis or at least 2 outpatient diagnoses of nAMD, DME, or RVO during the data year were included.14 Diagnoses were identified from the Medicare FFS claims based on the International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM) diagnosis codes for nAMD, DME, and RVO (Supplementary Excel File).15,16 Patients were classified to mutually exclusive cohorts based on the first diagnosis observed in the data year (ie, nAMD, DME, and RVO cohorts). Patient diseases were not required to be incident, and the length of time a patient had the disease of interest was not captured by the study data. Patients were not required to be on active treatment. The comparator cohort consisted of Medicare beneficiaries without any VTRDs, including nAMD, DME, and RVO.
Other inclusion criteria included survival through the data year. Individuals with VTRDs other than the conditions of interest or more than 1 VTRD were not included in this study. Examples of exclusionary diseases were endophthalmitis, central artery occlusion, severe glaucoma, surgery for glaucoma (eg, tube-shunt, trabeculectomy), proliferative diabetic retinopathy, congenital cataract, optic atrophy, amblyopia, optic neuritis, neuromyelitis optica, and ischemic optic neuropathy.
Outcomes
Outcomes were assessed from both MCBS questionnaires and Medicare claims data. ADLs, iADLs, and perceived visual function were collected from the MCBS responses. Difficulties with ADLs and iADLs were defined as stage 0 (no difficulties), stage I (mild), stage II (moderate), stage III (severe), and stage IV (complete) (Supplementary Table S1).17 Additionally, diagnosis of vision loss and anxiety were identified from Medicare claims based on ICD-9-CM or ICD-10-CM codes. Evidence of depression, falls, and fractures were identified by either diagnosis codes from Medicare claims or responses to corresponding MCBS queries.
Relationships between the outcomes of interest and baseline clinical and demographic characteristics were investigated. The characteristics examined included socioeconomic characteristics and overall health status derived from the MCBS responses, as well as Charlson Comorbidity Index (CCI) scores determined from Medicare claims with Quan’s adaptation (Supplementary Table S2).18
Statistical Analysis
The outcomes were compared between each disease cohort and the control cohort. For bivariate analyses, parametric F-test (mean) and nonparametric Wilcoxon rank-sum test (median) were used to compare continuous variables (eg, age, CCI scores) between 2 groups. Rao-Scott chi-square tests were used to compare binary variables (eg, presence of falls, fractures, and depression/anxiety) and categorical (eg, ADL/iADL status) variables among 2 or more groups.
Adjusted models were developed only when bivariate relationships were statistically significant. Logistic regression was used to assess differences in perceived visual function, diagnosed vision loss, and presence of falls, fractures, and depression/anxiety (ie, evidence of either depression or anxiety) between those with retinal diseases of interest and the control cohort. The association of the retinal disease of interest with ADL/iADL status was explored with multinomial logistic regression. For each such analysis, the covariate list was determined upon results of bivariate analyses that compared the corresponding disease cohort with the control cohort and expert opinions derived from clinical input and published literature. All models were adjusted for age, sex, race/ethnicity, and poverty status of Medicare beneficiaries. In the visual function model, additional covariates included the presence of cataract(s) and glaucoma; the additional covariates for the ADL/iADL status models included CCI categories, the presence of arthritis, dementia, hypertension, and osteoporosis.
Balanced repeated replication weights were used for variance estimation in the bivariate tests and regression models to account for nonindependence of the person-years in the multiple year pooled dataset, yielding pooled estimates that represent a moving average of nationally representative year-specific estimates. MCBS weights accounted for potential nonresponse and sample coverage bias. Annualized weighted Ns and percentages were reported to represent the Medicare population. The pooled estimates can be interpreted as being representative of the midpoint of the pooled period (ie, year 2012). The annualized weighted Ns were derived by dividing weighted Ns by the total number of data years (ie, 12) to represent the average number of Medicare beneficiaries in a given year.
A P value <.05 was considered statistically significant for all analyses. All analyses accounted for the complex survey design by using survey procedures with Statistical Analysis System version 9.4 software (SAS Institute Inc.).
RESULTS
Study Sample
A total of 2292 patients, representing more than 7 million of the weighted population, with nAMD, DME, or RVO during the data years of 2006 to 2013 and 2015 to 2018 were identified. Of these, 1580 (annualized weighted N = 430 924) fulfilled the eligibility criteria for the overall study cohort, including 1228 (annualized weighted N = 322 415) patients with nAMD, 101 (annualized weighted N = 34 074) with DME, and 251 (annualized weighted N = 74 436) with RVO (Figure 1A). For the control cohort, 104 088 (annualized weighted N = 34 494 243) beneficiaries fulfilled eligibility criteria (Figure 1B).
Unadjusted Demographic and Socioeconomic Characteristics
Patient characteristics differed between the nAMD, DME, and RVO cohorts and the control group (Table 1). The nAMD cohort was the oldest among the 4 cohorts studied (age in mean years [SE]: nAMD, 83.0 [0.4]; DME, 71.6 [1.5]; RVO, 78.8 [0.7]; control, 72.0 [0.1]), and the nAMD and RVO cohorts were both significantly older than the control cohort (P < .0001). Compared with the control cohort, the nAMD cohort had a significantly higher proportion of females (61.6% vs 55.7%, P = .0035), non-Hispanic White people (95.6% vs 83.0%, P < .0001), nonmetropolitan residents (28.3% vs 23.2%, P = .0031), and beneficiaries with income greater than 200% above the federal poverty line (60.1% vs 55.1%, P = .0029). The nAMD and DME cohorts included a significantly higher proportion of beneficiaries receiving veteran benefits than the control cohort (control: 5.2%; vs nAMD: 8.9%, P = .0001; vs DME: 16.6%, P < .0001). Use of anti-VEGF injections was significantly higher (P < .0001) in all 3 cohorts (n [%]: nAMD, 919 [74.6]; DME, 46 [48.0]; and RVO, 109 [41.9]) than in the control cohort (1504 [1.5]).
The burden of comorbidities was significantly higher among the nAMD, DME, and RVO cohorts compared with the control cohort (see Table 2). Rates of CCI scores of 3 or more for the nAMD, DME, and RVO groups were 26.4%, 54.3%, and 32.5%, respectively, compared with 12.8% for the control cohort. Most ocular comorbidities and many nonocular comorbidities occurred at a higher frequency in the nAMD, DME, and RVO cohorts compared with controls. The most prevalent ocular comorbidity across the retinal disease cohorts was cataracts, which were significantly more common in the nAMD, DME, and RVO cohorts (P < .0001; 60.6%, 68.5%, and 63.7%, respectively) than the control cohort (40.0%). Hypertension and cardiovascular disease were more prevalent in the nAMD, DME, and RVO cohorts than in the control cohort (hypertension [control 58.5% vs nAMD: 64.7%, P = .0011; vs DME: 76.5%, P = .0072; vs RVO: 70.2, P = .0101]; cardiovascular disease [control 37.6% vs nAMD: 68.3%, P < .0001; vs DME: 76.0%, P < .0001; vs RVO: 65.6, P < .0001]).
Vision Impairment and Functional Status
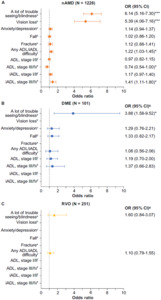
When adjusted for covariates, patients with either nAMD (odds ratio [OR], 6.14; 95% confidence interval [CI], 5.16-7.30; P < .0001) or DME (OR, 3.88; 95% CI, 1.58-9.52; P < .01) were more likely to report “a lot of trouble seeing/blindness” compared with controls (Figure 2). Patients with nAMD, but not DME or RVO, had higher odds of having diagnosed vision loss compared with controls, even after adjusting for other characteristics, such as the presence of a cataract or glaucoma (OR, 5.39; 95% CI, 4.06-7.16; P < .001). Although depression and falls were the 2 most commonly reported comorbidities and complications in all cohorts, after adjusting for covariates, the retinal diseases of interest were not significantly associated with the presence of anxiety/depression, fall, or fracture (Figure 2).
Patients with nAMD, but not DME or RVO, were more likely to report difficulty with ADL/iADL (OR, 1.22; 95% CI, 1.03-1.45; P < .05) (Figure 2). Before adjusting for covariates, the most frequently reported difficulty with individual ADLs was walking in all VTRD and control cohorts (control: 27.1%; nAMD: 33.1%, P = .0025; DME: 39.1%, P = .0376; RVO: 27.6% P = .9430) (Supplementary Excel File). However, when adjusting for covariates, none of the cohorts of interest were associated with having a statistically significant difference in ADL difficulty, although borderline significance was seen for having stage III/IV difficulty when comparing nAMD vs control (OR, 0.74; 95% CI, 0.54-1.00; P < .05) (Figure 2). Before adjustment, doing heavy housework was the most frequently reported difficulty in iADL across all 4 cohorts (control: 34.6%; nAMD: 44.4%, P <.0001; DME: 44.0%, P = .1924; RVO: 38.7%, P = .5302) (Supplementary Figure S1). When adjusting for covariates, patients with nAMD had a higher likelihood of having stage III/IV iADL difficulty (OR, 1.41; 95% CI, 1.11-1.80; P < .05) but not stage I/II difficulty (OR, 1.17; 95% CI, 0.97-1.40; P > .05) (Figure 2). There was no difference in stage III/IV and stage I/II between DME or RVO cohorts and the control group.
DISCUSSION
Topline Summary
To our knowledge, this analysis is the first to assess patient-reported outcomes at the population level (using the MCBS linked with Medicare FFS claims) for nAMD, DME, and RVO. These analyses highlight the association between the disease and quality of life among community-dwelling Medicare beneficiaries with nAMD and DME. Patients with nAMD or DME were more likely to report severe visual impairment compared with those without VTRDs, although only those with nAMD were more likely to be diagnosed with vision loss. It is possible that vision loss was under-recorded via diagnosis codes. Given the selection criteria used in this study (eg, ≥1 inpatient or 2 outpatient diagnoses, full-year enrollment, and exclusion of other vision-threatening diseases), the annualized weighted N related to each study cohort was much smaller than the number of Medicare patients diagnosed with VTRDs in 2019 (nAMD: 547 744, DME: 284 235; branched RVO: 159 882; and central RVO: 94 745).19
Impact on Quality of Life: ADLs, iADLS, and Complications/Comorbidities
Difficulty with heavy housework was the most commonly reported iADL for all cohorts. Unlike those in the DME and RVO cohorts, those in the nAMD cohort reported having more difficulties than those in the control cohort with using the telephone, shopping, managing money, and preparing meals. Loss of central vision can make it hard to use cell phones or read details on currency and packages, possibly explaining why these tasks could become more difficult for patients with nAMD. nAMD was associated with higher likelihood of reporting stage III/IV iADL difficulty, but not stage I/II difficulty. This may suggest the impact to a patients’ functional status may be relatively unaffected until central vision loss falls below a certain threshold, at which point the patient may experience severe loss of daily functional ability.
In previous research, patients with AMD were reported to experience more falls and injuries related to falls, with fear of falling contributing to a decrease in quality of life.4–6 In this investigation, about one-third of patients with nAMD, DME, or RVO in the Medicare population reported experiencing falls (33.3%, 41.4%, and 29.0%, respectively), compared with 27.7% of patients in the control cohort. However, after adjusting for age, sex, race/ethnicity, poverty status, comorbidities, and other relevant covariates, none of the retinal diseases of interest were associated with increased falls or fractures when compared with the control cohort. This may have been due to the relatively small number of patients to measure a relatively infrequent medical event or reflect real changes in outcomes arising from a combination of factors including better patient education, improved environments for seniors, and the increased use of anti-VEGF agents in the treatment of VTRDs since first approval in 2004 (shortly before the initial year from which our data were captured).20 Currently, intravitreal injections of anti-VEGF agents, generally given every 1 to 3 months, are the established standard-of-care treatment for these VTRDs and have been shown in clinical trials to be effective in improving or maintaining vision.21–23 With the use of these drugs in more recent years, the Medicare population may be experiencing less vision impairment overall than was the case at the time of the previous studies that did find an increase in falls and fractures; additional studies are needed to confirm this hypothesis.
In the past, patients reporting visual function loss have been shown to be at increased risk of depression.24 In this study, when compared with the control cohort, both anxiety and depression were more common in the nAMD cohort, and depression was more common in the DME cohort; however, neither retinal disease was associated with either anxiety or depression after adjusting for relevant covariates. This may be because of the mean age of patients being over 70 years for all cohorts in the current investigation since the association of depression and vision loss is stronger in younger working-age adults compared with those 65 years of age and older.7 In addition, in studies exploring the association of visual impairment and depression, depression was less commonly reported in studies designed to detect multiple disabilities (like the MCBS) than in those with the primary aim of detecting depression.25
Limitations
One of the most notable limitations of this investigation is that patient visual acuity outcomes are unknown, as the MCBS data have no linkage to electronic health records. Instead, vision loss was identified by a general self-reported assessment of vision difficulties or a diagnosis code, which may be underutilized in the real-world setting and used inconsistently across practices. Additionally, the cross-sectional design of this study does not ensure that visual function measures, ADLs, and iADLs (typically collected near the end of the year) were collected after disease diagnosis (which could occur any time that year). Given the focus upon disease burden, we did not seek to determine any relationship between exposure (ie, management of disease) and outcome. Since this study utilized the MCBS survey responses that were reported by Medicare beneficiaries or their proxies, it is prone to the limitations commonly observed in other studies based on survey data (eg, recall bias). Another limitation is that the sample sizes for DME and RVO cohorts were relatively small compared with the nAMD cohort, which did make it more difficult to detect significant differences. Since this study included a sample of Medicare beneficiaries enrolled in the FFS plans, the study results may not be generalized to Medicare beneficiaries enrolled in managed care plans. Similarly, the results may not be generalizable to patients who changed their insurance plans since full-year enrollment in Medicare Parts A and B was required for inclusion in this study. Further analyses of the MCBS data linked with claims are warranted to explore the role of treatments for nAMD or DME in improving perceived visual function and overall functional status in the Medicare population.
Implications
These findings suggest there is still significant disease burden in patients with VTRDs, especially nAMD. Patients with nAMD continue to experience more vision loss and reported a lot of trouble seeing and blindness significantly more often than patients in the control cohort even though most nAMD patients (75%) received anti-VEGF treatment. Potential reasons for this include poor responsiveness to existing treatment options seen in some patients with nAMD and high treatment burden due to the frequency of ongoing injections. A similar but nonsignificant trend was also observed in patients with DME and RVO. Solutions for improving vision and functional status in patients living with retinal diseases include facilitating greater patient education and care coordination to facilitate early screening of at-risk patients to prevent irreversible disease progression, as well as improving access to available treatments. Additionally, advanced interventions that improve effectiveness and reduce treatment burden may further reduce vision impairment associated with these retinal diseases.
CONCLUSIONS
Patients with nAMD continue to experience more vision impairment and worse functional status compared with a similar population of Medicare beneficiaries despite availability of therapies like anti-VEGF agents to treat retinal disease. However, when compared with the control cohort, none of the retinal diseases of interest were associated with increased falls, fractures, or anxiety and depression after adjusting for relevant covariates.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Disclosures
V.G., A.M., C.D.N., and I.M.A. are employees of Genentech, Inc. G.O., J.B., and D.B. have consultancies with Genentech, Inc. F.S.A. has consultancies with Allergan/AbbVie, Apellis, EyePoint, Genentech, Inc., and Regeneron and is on the speakers’ bureau of Apellis. A.M.K. has consultancies with 4D Molecular Therapeutics, AbbVie, Adverum, Aerie, Aldebaran, Allergan, Apellis, Applied Genetics Technologies Corporation, Arrowhead, Aviceda, Bausch + Lomb, Broadwing Bio, Clearside, Exegenesis Bio, EyePoint, Frontera, Genentech, Inc., Gyroscope, iLumen, Iveric Bio, Janssen, Kartos, Kato, Kodiak Sciences, Kriya, Nanoscope, Notal, Novartis, Ocular Therapeutix, Oculis, OcuTerra, Olives Bio, Opthea, Oxurion, Perfuse, PolyPhotonix, Protagonist, Ray Therapeutics, RecensMedical, Regeneron, Regenxbio, RevOpsis, Roche, Stealth, Thea, Unity, Vanotech, and Vial; has received funding and grants from 4D Molecular Therapeutics, Adverum, Annexon, Apellis, Genentech, Inc., Gyroscope, Iveric Bio, Kodiak, Neurotech, NGM Bio, Novartis, Ocular Therapeutix, Oculis, OcuTerra, Opthea, Oxurion, Regenxbio, Roche, and Unity; and reports stock ownership or options in RevOpsis. X.Z. and A.N. are employee of IQVIA. T.B. and K.W. were employees of IQVIA at the time of the study
Presentation
Portions of the data were presented at The Professional Society for Pharmacoeconomics and Outcomes Research (ISPOR) and Academy of Managed Care Pharmacy (AMCP) Nexus Meetings in 2021.

**_and_control_cohorts_**(b)**.png)
**_and_control_cohorts_**(b)**.png)
