INTRODUCTION
Arthritis is the leading cause of disability among adults in the United States; osteoarthritis (OA) is the most common form of arthritis, affecting approximately 32.5 million people worldwide.1 OA can cause pain, swelling, and stiffness in patients.2 As, unlike other forms of arthritis, there is no approved disease-modifying treatment that slows the progression of the disease, much of the clinical discussion surrounding OA is focused on managing pain and other related symptoms. Pharmacological treatments recommended by the American College of Rheumatology to manage symptoms of OA include topical and oral nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular glucocorticoid injections, intra-articular hyaluronic acid injections, duloxetine, acetaminophen, tramadol, and non-tramadol opioids.3
Although commonly prescribed in management of symptoms associated with OA, opioids are not recommended or only conditionally recommended in certain situations, such as when other treatments have failed.4 However, prior research has shown that their usage among patients with OA is more pervasive than the clinical recommendations would suggest.5,6 The conditional nature of the recommendation for opioid use by OA patients is based on a number of factors, including the side effects associated with OA use. While risks of addiction, abuse, and misuse are often a main focus of prescribers and patients, other common side effects can include drowsiness and dizziness. These side effects can lead to more serious adverse events, such as falls and subsequent fractures.7,8 Although other side effects of opioid prescribing use are well documented, including in patient populations with OA, falls and fractures are equally important clinical outcomes which remain less understood.9
Prior research has shown that a substantial proportion of older adults who are prescribed opioids experience falls and fractures following drug
initiation.10–12 Importantly, falls and fractures represent serious clinical events which impact patients’ quality of life and may even potentially increase mortality risk.13 In addition, among patients with OA undergoing total hip replacement, a history of falls prior to surgery has been shown to be associated with worse surgical outcomes, including increased rates of surgical revisions, 30-day and 90-day hospitalizations, postsurgical complications, and higher total healthcare costs (unpublished data).14 However, less is known about the effect of opioid use on types and frequencies of falls and fractures among the broader population of patients with OA of the hip and/or knee. Also, regarding the risk factors for falls and/or fractures and in the context of clinical comorbidities, there is limited published research that seeks to establish a comprehensive model of factors that influence fall and fracture risk among elderly patients.15,16 Given the gaps in evidence around these common adverse events associated with opioid use, this study sought to assess the frequency, time to event, and risk factors associated with a fall or fracture in a commercially insured US population of patients with OA taking opioids (ie, tramadol or non-tramadol opioids).
This study had 3 main objectives. First, analyses sought to assess and quantify the frequency and other characteristics associated with a fall or fracture among patients with OA of the hip and/or knee using real-world claims data. Next, time to fall or fracture following the prescribing of opioids was assessed using Kaplan-Meier analyses. In addition, this study also sought to develop a comprehensive model of risk factors associated with falls and fractures among patients with OA who were prescribed opioids, including prescribing of non-tramadol opioids, patient comorbidities, and other characteristics.
METHODS
Data Source
This study used deidentified administrative claims data from OptumHealth Care Solutions, Inc (Optum), a database containing healthcare utilization records for approximately 19 million privately insured lives (including employees, spouses, dependents, and retirees) from over 84 large, self-insured, US-based companies. The database contains information regarding patient age, gender, enrollment history, medical diagnoses, procedures performed, dates and place of services, prescription drug use, and payment amounts.
Sample Selection
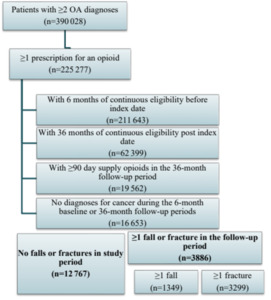
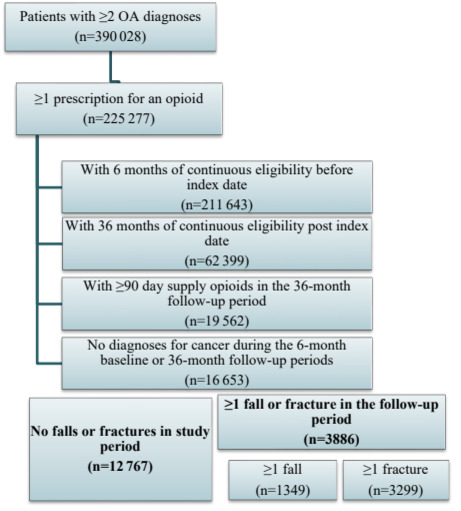
Patients with at least 2 diagnoses of OA of the hip and/or knee (according to the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD-9-CM, ICD-10-CM]) and who received at least 1 prescription for an opioid (according to generic product identifier [GPI] codes) during the study period were identified. The list of ICD-9-CM, ICD-10-CM, and GPI codes used are available upon request. The index date was defined as the date of the earliest opioid prescription occurring after each patient’s first OA diagnosis. Patients were required to be at least 18 years old on the index date and be continuously enrolled during the 6 months before (baseline period) and 36 months after (follow-up period) the index date to ensure that all relevant prescription and medical claims were captured. The index date was included in the 36-month follow-up period. Additionally, consistent with prior research on “chronic opioid use” and to help ensure that the prescription opioid(s) were prescribed for OA (as opposed to short-term, acute pain), patients were required to have at least 90 days of cumulative supply of opioids during the follow-up period.9 Lastly, patients were divided into 2 mutually exclusive cohorts based on if they had experienced a fall and/or fracture during the follow-up period: a fall/fracture cohort and a no fall/fracture cohort.
Study Design and Outcomes
This study consisted of a retrospective observational study of privately insured patients with OA in the United States. Characteristics in the baseline period and on index date were described using summary statistics, as were characteristics of falls and fractures in the follow-up period among patients with at least 1 fall or fracture. Next, time to first fall or fracture was assessed using Kaplan-Meier models. Finally, to assess risk of falls and fractures in patients taking opioids, controlling for confounding characteristics, logistic regression was used.
Multiple study outcomes and other variables were considered across analyses. To assess baseline health and sociodemographic characteristics of patients with OA with and without falls/fractures, the following characteristics were summarized at baseline or index date by cohort: age, gender, geographic region, Charlson Comorbidity Index (CCI),17 diagnoses of other comorbidities, type of OA diagnoses (eg, hip or knee), and index opioid prescription characteristics. Since physician specialty information is not available from pharmacy data, physician specialty on the most recent medical claim within 2 days of the index date was assessed.
Main study outcomes were defined from primary or secondary diagnoses codes for falls/fractures (ie, ICD-9-CM and ICD-10-CM codes) on the patient’s medical claim. In the 36-month follow-up period, the following characteristics were summarized among patients with at least 1 fall or fracture: rates of falls/fractures and rates of fall/fracture hospitalizations, rates of falls/fractures and hospitalizations by fracture location (ie, hip, spine, and other locations), type (ie, tramadol or non-tramadol opioids; immediate release [IR] or extended release [ER]) and dosage of opioids in the 30 days prior to a fall or fracture, and dosage of sedative-hypnotics in the 30 days prior to a fall or fracture. Fall/fracture hospitalizations were defined based on whether the diagnosis of fall or fracture was identified from an inpatient claim.
Outcomes used in time to event and Kaplan-Meier analysis considered a patient’s first fall/fracture. Time to a patient’s first fall, fracture, and combined fall or fracture, respectively, was assessed using Kaplan-Meier models. In addition to curves showing the percent of patients with an event over time, median time to each event and interquartile range (IQR) for time to each event were shown.
To assess the risk of experiencing a fall or fracture associated with type of opioid drug use and other clinical characteristics, logistic regression models were implemented to estimate odds ratios (ORs) with 95% CIs predicting this risk.
Statistical Analysis
Descriptive analysis: Descriptive statistics (mean, median, SD, percent) were used to summarize and compare baseline characteristics of the 2 cohorts (ie, fall/fracture cohort and no fall/fracture cohort). In addition, descriptive statistics were used to summarize the rates of falls/fractures, falls/fractures leading to hospitalization, and drug use characteristics prior to the first fall or fracture event during the follow-up period, among patients in the fall/fracture cohort.
During the baseline period to compare the two cohorts, statistical significance was assessed using the χ2 test for categorical variables and t tests for continuous variables. P values at the <.05 level were considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Time-to-event analysis: Kaplan-Meier time-to-event models were used to assess time to first event among patients with a fall or fracture. Due to the sample selection of patients with continuous eligibility and at least 1 fall/fracture, no censoring occurred and all patients in this analysis experienced an event over 36 months.
Risk factors analysis: Logistic regression models were used to assess risk factors for patients experiencing 1 or more fall or fracture in the follow-up period. Patients’ relative risk of a fall or fracture was assessed controlling for baseline comorbidities, baseline benzodiazepine use, age, region, OA diagnosis type (ie, hip vs non-hip), type of index opioid (eg, tramadol vs non-tramadol opioids), and index opioid dose (in terms of morphine milligram equivalents [MME]).18 Relative risk associated with a particular risk factor was expressed in ORs.
RESULTS
Sample Selection
The inclusion and exclusion criteria and the final sample counts are presented in Figure 1 by cohort. During the study period, 225383 patients were identified with at least 2 diagnoses of OA of the hip and/or knee who had at least 1 prescription for an opioid. 3886 of 16663 (23%) patients meeting the inclusion/exclusion criteria had at least 1 fall or fracture during the follow-up period (Figure 1). Of these patients, 1349 (35%) had at least 1 fall with an average of 1.4 fall claims, and 3299 (85%) patients had at least 1 fracture with an average of 5.3 fracture claims during the follow-up.
Baseline Characteristics
Patients with a fall or fracture were older on average than patients without a fall or fracture (66.2 vs 61.9 years old, P<.01) and also had a higher rate of patients that were female (72.1% vs 61.2%, P<.01) (Table 1).
Patients with a fall or fracture had higher rates of comorbidities at baseline than patients without a fall or fracture, as measured by CCI (0.6±1.0 vs 0.4±0.8, P<.01). In terms of comorbidities related to falls and fractures, patients in the fall/ fracture cohort had higher rates of balance impairment, fatigue, muscle weakness, osteopenia, osteoporosis, and prior falls (all P<.01). When considering the location of OA (hip and/or knee with or without OA in other joints), the ordering of prevalence rates per group was consistent across the cohorts, yet the fall or fracture cohort (vs the no fall or fracture cohort) had significantly fewer (P<.05) patients with knee only (45.2% vs 51.3%); more patients with knee and other joints (16.6% vs 13.6%), hip only (14.3% vs 13.0%), hip and other joints (4.5% vs 3.6%), and hip, knee, and other joints (1.4% vs 1.0%); and no significant differences in the rate of patients with hip and knee OA without other affected joints (3.0% vs 2.5%).
Patients who experienced a fall or fracture also had different drug utilization characteristics on the index date in comparison with patients without these events. Significantly more patients with a fall or fracture claim received prescriptions for non-tramadol opioids on the index date, both IR (72.2% vs 69.6%, P<.01) and ER (10.8% vs 8.8%, P<.01), and also had higher index doses in MME (60.3±186.9 vs 51.6±106.5, P<.01). Patients with a fall or fracture had similar rates of physician specialty (ie, primary care physician, orthopedist, or rheumatologist) on index date compared with patients without a fall or fracture (Table 1).
Descriptive Characteristics
In the 3-year follow-up period, substantially more patients in the fall/fracture cohort experienced at least 1 fracture (84.9%) than experienced at least 1 recorded fall (34.7%). Among those with at least 1 fall, patients experienced an average of 3.0 (±2.1) falls over the follow-up period, and 27.7% of those falls were associated with a hospitalization. Among the 3299 patients with at least 1 fracture in the follow-up period, the average number of fractures was 8.3 (±11.6), 1052 patients (31.9%) experienced a fracture associated with a hospitalization, and 13.5% of all fractures were associated with a hospitalization (Table 2).
The most common location of fracture was non-hip/non-spine (72.6%), followed by spine (15.8%), and then hip (12.5%). Patients experienced the greatest number of non-hip/non-spine fractures on average (7.7±11.1), followed by hip (7.0±7.6) and spine (6.1±7.7). Hip fractures tended to be the most severe, as 35.7% of all hip fractures were associated with a hospitalization, followed by spine (20.4%) and non-hip/non-spine (12.2%). However, the largest proportion of patients (19.9%) experienced at least 1 hospitalization associated with a non-hip/non-spine fracture when compared with hip (8.5%) and spine (6.2%) (Table 2).
Over half the fall/fracture cohort had at least 1 prescription for an opioid in the 30 days prior to a fall or fracture (56.9%).The most commonly used opioids were IR non-tramadol opioids (43.6%), IR tramadol (12.6%), and ER non-tramadol opioids (11.5%). In the 30 days prior to a fall or fracture, patients had an average cumulative opioid dose of 2376.7 MME (±12 863.4). A small number of patients also had at least 1 prescription for benzodiazepines in the 30-day period prior to fall or fracture (14.4%) (Table 2).
Time to Event
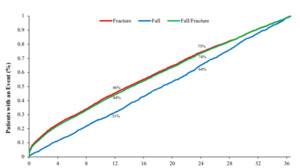
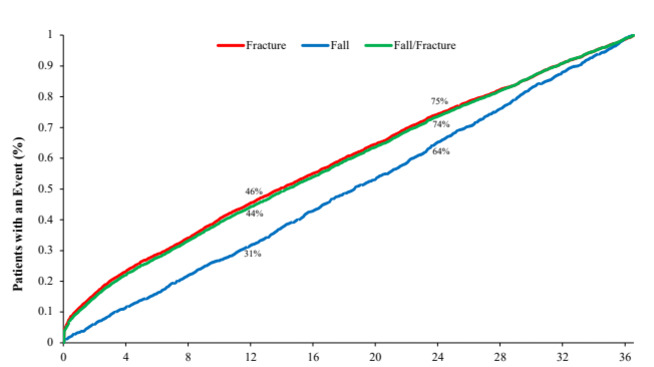
Among patients with either a fall or a fracture, the median time to first fall or fracture event was 14.3 months (IQR, 4.9- 24.7). Among patients with a fall, the median time to event was 18.6 months (IQR, 9.2-27.6), and among those with a fracture it was 13.9 months (IQR, 4.6-24.3). At 12 months after the index date, 44% of patients with either a fall or fracture in follow-up had experienced an event, 31% of patients with a fall in follow-up had experienced a fall, and 46% with a fracture had experienced a fracture. At 24 months after the index date, these rates were 74%, 64%, and 75% for the combined fall/fracture outcome, falls alone, and fractures alone, respectively (Figure 2).
Risk Factor Model Results
Table 3 reports factors identified as associated with a fall or fracture during the 36-month follow-up period. Even controlling for underlying demographics and other baseline characteristics, use of prescriptions of non-tramadol opioids had an estimated OR of 1.31 (P<.01), and a dosage increase of 10 MME (ie, patients prescribed higher doses) on index had an estimated OR of 1.01 (P<.01).
Controlling for other baseline characteristics included in the regression, the other clinical characteristics that were significantly (P<.05) associated with increased risk of experiencing a fall or fracture were for diagnosis of alcohol use (OR, 3.41), history of falling (OR, 2.19), diagnosis of osteoporosis (OR, 2.06), diagnosis of Parkinson’s disease (OR, 1.90), diagnosis of balance impairment (OR, 1.40), and benzodiazepine use (OR, 1.21). In addition, older age was associated with increased risk (OR, 1.03). Males were at a significantly decreased risk of a fall or fracture (OR, 0.69) compared with females.
DISCUSSION
The study considered recent, real-world prescribing of opioids to a large sample of commercially insured patients with OA of the hip and/or knee. Opioid use is known to increase the risk of falls and fractures in at least 3 distinct ways, including effects on the central nervous system such as sedation and dizziness, reduced bone density by direct effects on osteoblasts, and hypogonadism induced by chronic opioid use.19 Given these effects, the main goal of this study was to assess the frequency, characteristics, and time to event for falls/fractures. In addition, this study also assessed risk factors associated with a fall or fracture in this patient population. At baseline, patients with OA experiencing falls and fractures had significantly higher rates of comorbidities related to falls and fractures, including balance impairment, fatigue, muscle weakness, osteopenia, osteoporosis, and prior falls. Substantial increases were estimated in the frequency of falls/ fractures during the follow-up period after patients were prescribed opioids, highlighting a possible role of drug side effects like drowsiness or dizziness in contributing to adverse events in patients with OA. Among patients with OA meeting the inclusion criteria, approximately 1 in 5 experienced a fall or fracture in the 36-month follow-up period after their first prescription. Study findings also provide additional evidence around the clinical and economic burden of these negative clinical outcomes associated with opioids. For example, 27.7% of all observed falls and 13.5% of all observed fractures were estimated to be associated with an inpatient hospitalization.
Findings from time-to-event analyses highlight the additional need for continuous, long-term observation and clinical management of OA of the hip and/or knee in patients taking opioids by rheumatologists and other clinicians involved in primary care. Among patients experiencing an event, median time to fall and fracture were 18.6, and 13.9 months, respectively. Physicians may increasingly recognize that there is no time point after which patients prescribed opioids for the treatment of OA-related pain are at decreased risk for negative clinical outcomes associated with these medications.9
To our knowledge, this study is among only a few that clearly identifies the substantial impact and frequency of falls and fractures associated with prescribing of non-tramadol opioids to patients with OA.9,20 We estimate that 57% of patients with OA who were prescribed opioids at index and experiencing a fall or fracture had an opioid prescription in the 30 days prior to the event. The most common type of opioid prescribed during this period were non-tramadol IR opioids, with 44% of fall/fracture patients having at least 1 prescription. These and other findings are supportive of recently updated clinical guidelines from the American College of Rheumatology, which conditionally recommend against prescribing of non-tramadol opioids for patients with hip and knee OA.21 Although opioids can help alleviate pain associated with OA, our analysis, based on observed prescription and medical claims, found that patients with OA of the hip and/or knee who were prescribed opioids are at a high risk for falls and fractures over the next 3 years.
Given the substantial clinical burden imposed by a fall or fracture for a patient with OA, risks of falls/fractures should be an important consideration in the ongoing treatment of patients with OA.22 Findings from the analysis of risk factors suggest successful clinical management of OA in key patient subgroups, such as patients with comorbid Parkinson’s disease, osteoporosis, or other comorbid conditions, may have benefits in terms of reduced falls and fractures. However, controlling for other characteristics, no significant differences in risk were observed for patients with OA of the hip (alone or in combination with other locations) and OA of the knee (alone or in combination with other, non-hip locations). Drug use characteristics of patients experiencing falls and fractures described in this study (Table 2) also highlight that these negative clinical outcomes may be a manifestation of ineffective treatment due to concomitant drug use and switching of therapies.
Limitations
This analysis, consistent with claims analyses more generally, have certain inherent limitations. Underlying data reflect a privately insured patient population and may not be representative of other payer populations, such as Medicare or Medicaid. Identification of patients is reliant on algorithms and may not be perfectly accurate in selecting patients taking opioids for OA use; despite the 90-day drug use threshold for study inclusion, patients may be taking opioids for other reasons. The analysis cannot ensure that patients are using the treatments for OA itself, although the fact that patients are diagnosed with OA and the use of these treatments after the initial OA diagnosis is suggestive.
Analysis relied on the accuracy of diagnosis codes to identify patients with OA and their comorbidity profiles at baseline. Additionally, diagnosis codes for fall events that resulted in medical interventions, in particular, may lack specificity and may underreport the true prevalence of falls in this population.23 Approximately 30% of falls among the elderly are estimated to result in medical intervention.24 Any miscoding along these lines will affect the results.
By considering the 3-year period after the patient with OA’s first opioid prescription, results are able to better identify the associated impact of prescription drug treatment independent of any baseline comorbidities. However, certain potential confounding characteristics that may have an effect on risk of experiencing a fall or fracture, such as progression of OA disease severity, were not included, and results from the risk analysis should not be interpreted as causative factors for falls/fractures.
CONCLUSIONS
This study provides new evidence regarding the frequency, characteristics, and statistically significant risk factors contributing to falls/fractures in patients with OA of the hip and/or knee in the United States. Results provide additional understanding as to the extent of negative clinical outcomes associated with prescription opioid medications, in addition to risks of addiction, abuse, and misuse more commonly considered in clinical assessment. Findings also suggest an ongoing unmet need for non-opioid medications to address OA-related pain in patients without imposing significant risk of falls and fractures. Risks of falls/fractures should be an area of increased focus for physicians in clinical assessment and patient education when managing OA-related pain with opioids.
Funding
This study was funded by Pfizer and Eli Lilly and Company.
Disclosures
S.S. is a paid consultant to Pfizer and Eli Lilly and Company in connection with this study. B.R., A.W., and W.P. are employees of the Analysis Group, who were paid consultants to Pfizer and Eli Lilly and Company for this study and development of this manuscript. R.R. is an employee and stockholder of Eli Lilly and Company. P.S., C.B., B.E., and S.T. are employees of Pfizer with stock and/or stock options.
Meeting Presentation
This study was presented as a poster at ISPOR and appeared in abstract form in Value Health. 2021;24(suppl 1):S138.