INTRODUCTION
Recent improvements in the assessment and management of pediatric pain include the Joint Commission on Accreditation of Health Care Organizations 2001 mandate to treat and assess pain in all patients and increased inclusion of pediatric patients in drug development studies.1 However, despite these improvements, 60% of hospitalized children undergoing surgery experience at least 1 day of moderate-to-severe pain after surgery.2 In particular, pediatric patients undergoing spine surgery are likely to experience postsurgical pain, which may in turn affect exposure to opioids, length of stay (LOS) in the hospital, and medical costs.3,4
Despite the associated opioid-related adverse events (ORAEs), opioids are commonly prescribed for pain management following pediatric surgery.5–9 Prescription of opioids can be associated with misuse and development of persistent opioid use, particularly in adolescents, and ORAEs that may lead to substantial health-care resource utilization.5,10 From 1997 to 2012, the incidence of hospitalizations for prescription opioid poisonings increased nearly 2-fold among children and adolescents aged 1–19 years, with the highest overall incidence reported in adolescents (aged 15–19 years).11 Multimodal analgesic regimens, which include nonopioid regional anesthetics, are being implemented by hospitals to not only better manage postsurgical pain and improve recovery, but also to minimize in-hospital postsurgical opioid consumption.12
Liposomal bupivacaine (LB) is a long-acting single-dose infiltration multivesicular liposome formulation of the local anesthetic bupivacaine that provides prolonged bupivacaine release over several days.13 Adult patients treated with LB demonstrated reduced postsurgical pain and opioid consumption when administered LB via local infiltration or as an interscalene branchial plexus nerve block in multiple surgical procedures.13 However, use of LB to treat postsurgical pain in orthopedic surgery has rarely been assessed in pediatric patients. Data are also lacking regarding the effectiveness of LB on hospital cost of care in these patients. This retrospective analysis aimed to comprehensively assess both opioid-related and economic outcomes, including opioid consumption, ORAEs, hospital LOS, and total hospital costs, associated with the use of LB or non-LB analgesia in pediatric patients undergoing primary spine surgery.
MATERIALS AND METHODS
Study Design
We performed a retrospective analysis of the deidentified Premier Healthcare Database, which contains administrative data since January 2000 from >1000 US hospitals and health-care systems, including academic and nonacademic institutions in both urban and rural locations.14 Over 231 million unique patients representing ~25% of US inpatient admissions are included in the database. The database contains information on hospital characteristics, patient visit characteristics, specialties of admitting and attending physicians, health-care payers, and patient information such as demographics, disease states, costs for billed services, and patient discharge health status obtained from standard hospital discharge billing. This analysis was exempt from institutional review board review requirements per Department of Health and Human Services policy (Title 45 Code of Federal Regulations, Part 46 of the United States) because patient records were deidentified.
Patient Population
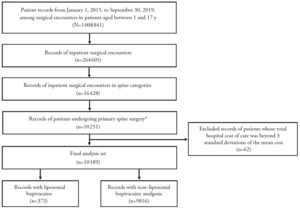
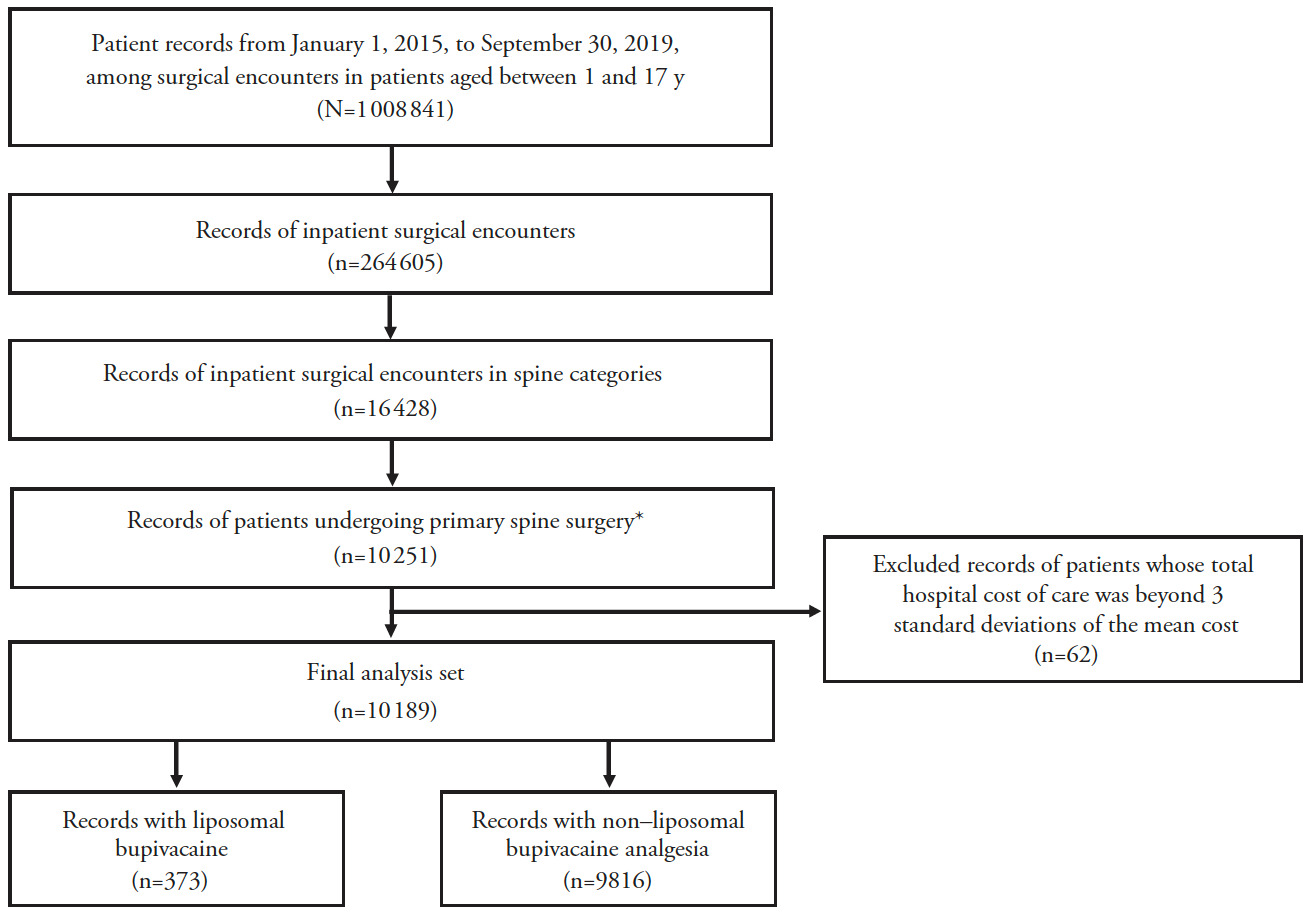
Data were analyzed from pediatric patients aged 1–17 years who underwent inpatient primary spine surgery including discectomy, lumbosacral fusion, other fusion, laminectomy, or other spine surgery, which were identified by International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes (Supplemental Table 1) between January 1, 2015, and September 30, 2019, and received either LB analgesia as identified by the Premier Healthcare Database standard charge master codes (Supplemental Table 2) or who did not receive LB analgesia for pain management following the procedure (Figure 1). Patients were excluded if they had multiple records of the same primary surgery admission or if their hospital costs were ≥3 standard deviations beyond the mean cost because they were considered outliers. The follow-up time for the patients covered the entire hospitalization period from admission to discharge.
Study Variables
The primary treatment exposure variable in this study was the use of LB as a binary variable, generated by the Premier Healthcare Database standard charge master codes. The primary outcome of interest was inpatient opioid prescription, which was extracted from standard charge master codes for opioids and was converted into total morphine milligram equivalents (MMEs). Additionally, LOS in the hospital, reported in days, and total cost of hospital care, in US dollars, were extracted from the Premier Healthcare Database hospital encounter summary. The other secondary outcomes of interest were ORAEs, including cardiovascular, central nervous system, gastrointestinal, respiratory, skin, and other complications, defined by ICD-9 and ICD-10 diagnosis codes, using a previously published approach.15
The patient demographic information comprised age (in years) at hospital admission, sex, race (White, Black, other), Quan-Charlson Comorbidity index defined by ICD-9 and ICD-10 diagnosis codes and recorded at hospital admission prior to surgery, patient-controlled analgesia (yes, no), surgery type (discectomy, lumbosacral fusion, other fusion, laminectomy, other spine surgery), and surgical year (2015–2019). The hospital characteristics included location (urban, rural), hospital size (0–299, ≥300 beds), hospital teaching status (yes, no), and geographic region (northwest, southwest, south, west).
Statistical Analyses
Continuous variables were summarized using the mean and standard deviation, and categorical variables were summarized using the frequency and percentage. A generalized linear model was used to compare LB versus non-LB analgesia in relation to clinical (opioid consumption during hospital stay in MMEs [conversion factors for morphine equivalents are provided in Supplemental Table 3] and ORAEs) and economic (LOS and total hospital costs) outcomes for all inpatient spine surgery types combined, assuming gamma distribution with log link function for MME and cost outcomes, negative binomial distribution with log link function for LOS, and binomial distribution with logit link function for ORAEs. To control for patient and hospital characteristics, the analytic model was adjusted for age, sex, race, Quan-Charlson Comorbidity Index, location, teaching hospital status, hospital size (bed number), geographic region, use of patient-controlled analgesia, and surgery type. All statistical analyses were performed using SAS software, version 9.4. A P value of <0.05 was considered statistically significant.
When the modeling distribution for total MME intake was tested using the family test with ordinary least squares method, the gamma distribution appeared to be valid with an observed gamma of 2.14 (95% confidence interval [CI], 2.08–2.19). Although the test for total cost of care seemed to suggest Poisson distribution (observed gamma of 1.02 [95% CI, 0.94–1.11]), we observed very similar results to our original analysis when Poisson distribution was applied. For the ORAEs outcome, logit link appeared to be appropriate with the goodness of link assessment (observed parameter, 0.03 [95% CI, <0.001–1.18]).
RESULTS
Patient Characteristics
Of >2 million patients screened, 10 189 pediatric patients were identified for this analysis, with 373 patients in the LB cohort and 9816 in the non-LB cohort (Figure 1). Generally, baseline characteristics were comparable between the LB and non-LB cohorts, although the LB cohort appeared to be older than the non-LB cohort (mean age, 14 and 12 years, respectively; Table 1).
Approximately 60% of patients in each treatment cohort were female, and approximately two-thirds of patients in each treatment cohort were White; the mean Quan-Charlson Comorbidity Index was 0.2 and 0.3 for the LB and non-LB cohorts, respectively. Less than 1% of patients (n=98) were identified as receiving epidural analgesia. Baseline hospital characteristics and year of surgery were mostly similar for both treatment cohorts. Spinal fusion was the most common surgery in both the LB (79%) and non-LB (57%) cohorts.
Clinical Outcomes
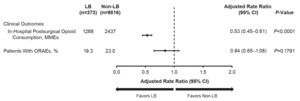
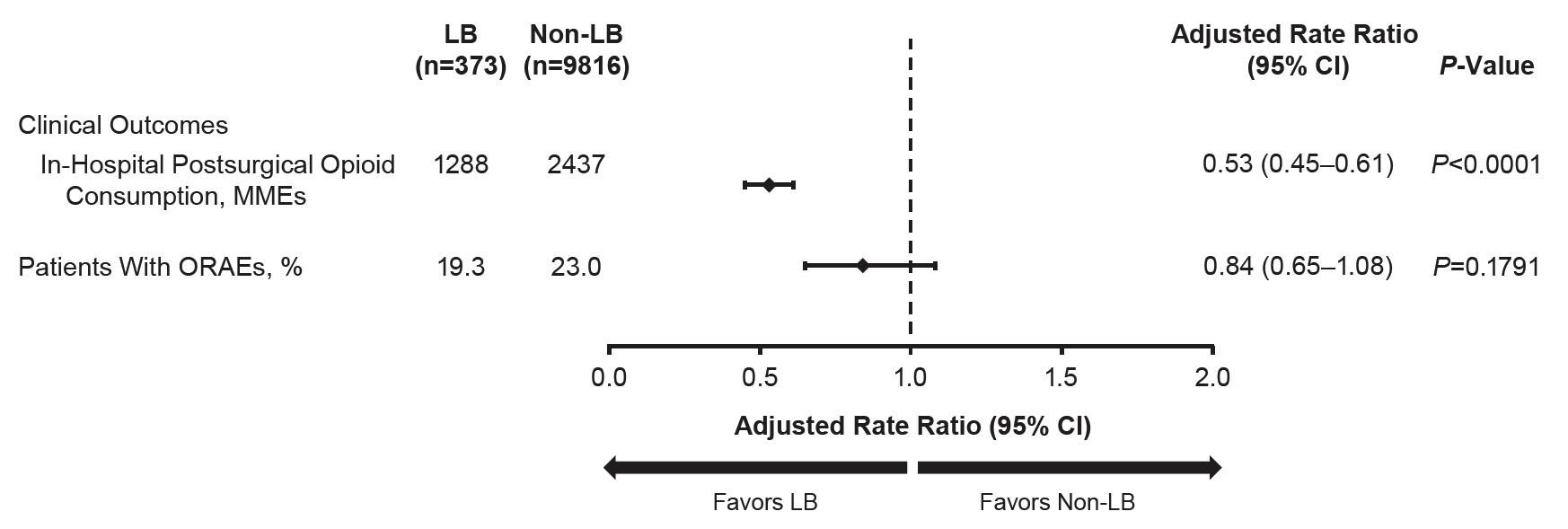
Overall, the LB cohort consumed 47% fewer in-hospital postsurgical opioids than the non-LB cohort (adjusted MMEs, 1288 vs 2437; P<0.0001) (Figure 2). Across all procedures, the proportion of patients who experienced ORAEs was not significantly different between the LB and non-LB cohort (adjusted proportion, 19% vs 23%; P=0.1791). Unadjusted values are provided in Supplemental Tables 4 and 5.
Economic Outcomes
Overall, the LB cohort demonstrated a 14% shorter LOS compared with the non-LB cohort (adjusted LOS, 3.5 vs 4.0 days; P=0.0003) (Figure 3). Patients receiving LB analgesia had significantly lower total hospital costs than patients receiving non-LB analgesia, corresponding to 8% lower total costs with LB (adjusted total cost, $29 790 vs $32 284; P=0.0227). The cost savings with LB versus non-LB analgesia were mostly attributable to hospital stay cost by room and board (ie, adjusted hospital stay cost, $6312 vs $7395; 15% savings) and central supply cost (ie, adjusted equipment cost, $8267 vs $9370; 12% savings). Unadjusted values are provided in Supplemental Table 6.
DISCUSSION
In this retrospective database analysis, pediatric patients with low mean Quan-Charlson Comorbidity Index16 undergoing inpatient primary spine surgery who received LB analgesia after surgery had statistically significant lower in-hospital opioid consumption, shorter hospital LOS, and lower total hospital costs compared with those who received non-LB analgesia. These clinical and economic benefits are in accordance with and expand upon results from other retrospective studies examining the use of opioid consumption and LOS for pediatric patients receiving LB or non-LB analgesia for postsurgical pain after spine deformity surgery and LOS for adult Medicare patients undergoing total knee arthroplasty.17,18
Adolescents (defined by the World Health Organization as those aged 10–19 years)19 may be at increased risk of opioid misuse compared with adults. This may be explained by changes in brain reward and habit formation centers during this developmental period.5,20 Exposure to prescription opioids can also contribute to potential opioid misuse and development of persistent opioid use in adolescents. Diversion of prescription opioids in the form of selling, trading, and loaning is common in adolescents, playing a critical role in opioid misuse.21 A retrospective analysis of postsurgical opioid refills found that ~5% of opioid-naive adolescents and young adults who filled an opioid prescription after surgery continued to fill their prescription >90 days after surgery; risk factors for persistent use included older age, female sex, and presurgical chronic pain.5 Similarly, another retrospective analysis found that 74% (N=346 251) of opioid-naive patients undergoing surgery aged 13 to 21 years filled an initial surgery-related opioid prescription. In the same study, patients who had a family member with long-term opioid use filled an opioid prescription 91 to 180 days after the procedure at a higher rate than those who did not.6 Given these previous reports of adolescent persistent opioid use, findings from the current study suggest that use of LB for postsurgical pain management may offer clinical benefits because it was associated with reduced in-hospital postsurgical opioid consumption after spine surgery. Together, use of LB may be beneficial in multimodal pain management strategies aiming to reduce postsurgical opioid exposure and risk for potential opioid misuse and dependence, especially in young patient populations.
In the current study, patients who received LB experienced shorter in-hospital LOS than those who received non-LB analgesia. This finding is consistent with previous reports in adult22,23 and pediatric17 patients undergoing spine surgery, as well as studies in adult patients in other surgical areas.24,25 Use of opioids in the postanesthesia care unit may contribute to ORAEs, possibly resulting in an increase in LOS and total costs.26,27 In the current study, lower opioid consumption along with numerically fewer ORAEs might have contributed to the shorter LOS seen in the LB group, as indicated by Olbrecht and colleagues.4 In addition, shorter LOS with LB has previously been hypothesized to be associated with earlier mobilization and activity, potentially due to increased pain control, in adult patients after lumbar spine surgery or total hip arthroplasty.23,25 However, because time to ambulation was not assessed, its contribution to reduced LOS in the current study cannot be determined.
A previous report has shown that intensive care unit and inpatient room costs contributed greatly to overall hospital costs following idiopathic scoliosis correction surgery in adolescent patients.28 In the current study, LB was associated with shorter LOS and lower hospital costs compared with non-LB analgesia in pediatric patients undergoing spine surgery. Because the LB cohort had lower adjusted room and board costs than the non-LB cohort, these data suggest that use of treatments, such as LB, might reduce hospital LOS and subsequently health-care costs. Moreover, the LB cohort had lower adjusted costs for central supply than the non-LB cohort, suggesting that additional cost savings outside the hospital room may also factor into overall health-care cost savings. Cost of complications contributed by postsurgical opioid use and patient recovery experience are critical in drafting guidelines for cost-effective postsurgical patient care.29 LB potentially can reduce pain and the need for supplemental postsurgical opioids, thereby reducing pain and opioid-associated expenses, as well as improving patient satisfaction with postsurgical care.
LIMITATIONS
There are several limitations to consider when interpreting the present findings. One limitation is the potential underreporting of LB use in the database, thus limiting the generalizability of these findings. Fewer than 1% of patients in the entire study cohort were identified as receiving epidural analgesia for postsurgical pain, which is likely an underestimation due to challenges in finding this information among bundled payments in the database, making it difficult to control for non-LB analgesics that were used. The Premier Healthcare Database also does not provide information about perisurgical pathways, which may be heterogenous across institutions; as such, use of LB in spine surgery may reflect implementation of comprehensive enhanced recovery pathways in pediatric and adult patients that may have contributed to the reduction in opioid consumption and shorter hospital stays observed in the present study.17,30 Further, as the Premier Healthcare Database is an administrative database lacking clinical details, surgical information related to management protocols and practices was not available. We also did not have data on baseline MME intake, and thus, we were unable to control for this confounding factor in our model. Although the retrospective observational design of this analysis cannot adjust for all confounding variables, this analysis does provide real-world insight into clinical and economic outcomes that are otherwise challenging to assess across randomized controlled trials. Also, we cannot rule out the possibility that the differences we observed could be explained by confounding factors that are not available in the Premier Healthcare Database, including preexisting conditions, prior health-care use, or laboratory results. In addition, this study included several different spine procedures, which could contribute to the heterogeneity of the findings, although we have accounted for surgery types in the regression model. Finally, we were unable to identify whether specific postsurgical pain management protocols were used in the hospitals, which potentially could have influenced the amount of medications used as well as the multimodal approaches included.
CONCLUSION
In summary, pediatric patients undergoing inpatient primary spine surgery who received LB analgesia experienced improved outcomes compared with patients who did not. The LB cohort showed significant reductions of in-hospital postsurgical opioid consumption, LOS, and costs compared with those who received non-LB analgesia with no significant difference in ORAEs between cohorts. This retrospective observational analysis provides support for the use of LB as part of a multimodal analgesia regimen to manage postsurgical pain and mitigate clinical and economic outcomes in pediatric patients undergoing spine surgery. Future studies confirming these findings in larger sample sizes and in other pediatric surgical procedures are warranted.
Acknowledgments
The authors thank Mary Helen Tran for scientific inputs on result interpretation, and John Olczak for support in statistical programming and analysis.
Funding
This analysis was funded by Pacira BioSciences, Inc. Editorial assistance was provided under the direction of the authors by MedThink SciCom with support from Pacira BioSciences, Inc.
Author Contributions
(I) Conception and design: Jennifer H. Lin and Jessica Cirillo; (II) Administrative support: Jennifer H. Lin; (III) Provision of study materials or patients: Jennifer H. Lin and Jessica Cirillo; (IV) Collection and assembly of data: Jennifer H. Lin; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Conflicts of Interest
RTB is a paid consultant for Pacira BioSciences, Inc. and has received research funding from Pacira BioSciences, Inc. JS is a consultant for Pacira BioSciences, Inc. RG is a consultant for Stryker Spine and Orthopediatrics. JHL and JC are employees of and may hold stock options in Pacira BioSciences, Inc.
Ethical disclosure
This analysis was independently performed by Pacira BioSciences, Inc. using data from the Premier Healthcare Database. Because this analysis included only deidentified medical records, this study was considered exempt from institutional review board requirements per Department of Health and Human Services policy (Title 45 Code of Federal Regulations, Part 46 of the United States).