BACKGROUND
Human papillomavirus (HPV) infections are the etiologic agents of GW. More than 130 different types of the virus have been identified and divided into two groups according to epidemiological association with cervical cancer.1 The low-grade HPV group includes types 6 and 11, which are estimated to cause approximately 90% of GW cases.1,2 The high-risk HPV group, including types 16 and 18, causes precancerous lesions such as cervical intraepithelial neoplasia, cervical cancer, and anogenital cancer.3
HPV is one of the most frequent sexually transmitted viral infections, and nearly 65% of individuals with partners who have GW also develop GW.4,5 An estimated 6.2 million persons are newly infected every year in the United States alone, but most infections are asymptomatic or subclinical and become undetectable over time.6
Data on national GW incidence by country is limited, and prevalence estimates by country range widely, from 1.4% (Spain) to 25.6% (Nigeria).7–9 In Russia, as in many other countries, most available literature focuses on the oncogenic HPV type, and overall GW prevalence data are scarce. Bogdanova et al. evaluated the epidemiology of viral STIs from 2000 to 2011 in the Russian Federation and concluded that the total rates of genital herpes simplex virus infection remained constant (mean rate 19.8±1.4 per 100 000) while the total HPV infection rate during the same period increased from 27.4 to 29.2 per 100 000, with maximum reported rates of 34.7 in 2009.10
The large economic burden of GW treatment and management weighs heavily on a health care system. With rates of newly diagnosed GW cases increasing, the economic burden is also likely to increase, as even self-resolved GW cases can recur and in some cases are resistant to treatment. Currently available GW treatments include patient-applied (home-based) chemical treatments (podofilox, imiquimod), provider-administered (office-based) chemical treatments (podophyllin, trichloroacetic acid, interferon), and ablative treatments (cryotherapy, surgical removal, laser treatment).11
Although this study looks at the prevalence and healthcare utilization of GW within the Russian population, it can be generally noted that an increasing prevalence of GW incurs increased healthcare utilization resulting in higher economic cost. A study that looked at incidence and economic burden of GW in Korea showed that the total cost of outpatient clinics in 2015 was approximately $9.3 million.12 Another study that looked at GW in the United Kingdom estimated over 220 000 cases of GW in 2012, resulting in £58.44 million with £265 per patient.13 In 2008, Hillemanns et al. estimated overall third-party payer GW costs at €49.0 million and total societal costs at €54.1 million in Germany.14 In Spain, Castellsagué et al. estimated overall third-party payer GW costs at €47 million and total societal costs at €59.6 million in 2009.7 A recent study assessing the incidence and economic burden on the US commercially insured population reported estimated costs at $760 per 1000 individuals in the general population in 2004, with total costs exceeding $220 million.15 An Australian study showed an annual incidence of 2.19 cases of GW per 1000 Australians (95% confidence interval [CI]: 1.88 to 2.49). In addition, the estimated cost of managing GW annually was over US$14 million, with an estimated cost per treated case of US$251 for men and US$386 for women.16 To date, little research has been conducted to analyze GW incidence and prevalence in Russia. Data available in Russia focuses predominantly on cervical cancer. The country-specific, overall high-risk HPV frequency in republics of the former Soviet Union was estimated at 33.4%, and HPV 16 was found to be the most prevalent type in Russia (205/1967 women).17 A study of female patients aged >30 in St. Petersburg indicated that high-risk HPV was present in 13% of the study population.18 As such, the likelihood of GW caused by HPV and the significant economic impact on society may be higher than estimated. Given the lack of available data in Russia, the current study was designed to estimate GW prevalence in physician practices and GW-related health care resource use in Russia among male and female patients aged 18–60 years.
METHODS
Study Design
This was a cross-sectional study conducted through physician surveys in major federal districts in Russia, including Central (Moscow, Reutov, Mozhaysk, Yaroslavl, Belgorod, Voronezh, Lipetsk), Privolzhskiy (Penza, Saransk, Ulyanovsk, Orenburg, Izhevsk), Sibirskiy/Ural (Novosibirsk, Chelyabinsk, Tyumen), and Northwest (Baltiysk, Kaliningrad, St. Petersburg).
The study period was from January through June 2012. Ethical approval was obtained from an accredited external Institutional Review Board (IRB) for those sites not covered by their own internal IRB. The ethical conduct of this study was performed in accordance with the Declaration of Helsinki and the principles of Good Clinical Practices.19
Study Instruments. Two study instruments, including a physician survey and a two-week daily log, were used. The 30-minute physician survey posed questions related to resource use as part of the usual course of diagnosis, treatment, and follow-up care (inpatient, outpatient) for typical GW patients in participating physicians’ practices. The survey was completed by physicians who attended to and treated GW patients. The survey also included questions related to practice referral patterns, from general practice physicians to specialists as well as between specialists.
The physician two-week daily log recorded the number of all-cause, newly diagnosed, and existing GW patients (i.e., GW was the primary reason for the visit or GW was diagnosed during the visit), patients retained versus referred to other specialists for treatment, and patient age and gender information for all patients seen.
Inclusion and Exclusion Criteria. Participating physicians were identified through a Clinical Research Organization representative. The investigator list was compiled before study initiation and based on a database of investigators who participated in other clinical trials, an investigator list of those contacted during feasibility phases of other clinical studies, and physician and hospital contact information from open sources. The list contained an equal number of physicians in all three specialties from different cities.
Physicians included in this study (a) were specialists (obstetrician/gynecologists [OB/GYN], urologists [URO], and dermatologists [DERM] with 2–35 years of practice experience) that provided informed consent to participate, (b) devoted ≥30% of their time to treating patients for outpatient visits three or more work days per week (as opposed to inpatient surgeries, teaching or other activities) and spent 2 or more work days seeing patients for outpatient visits, (c) treated ≥50 patients for outpatient visits in a typical week, and (d) treated ≥50% of patients aged 18–60 years for outpatient visits.
Practice settings of participating physicians included private office/clinic, private hospital, and public health care center/clinic or hospital (including university and military hospitals). There were no exclusion criteria for physicians participating in the study.
Prevalence and Health Care Resource Use. GW prevalence was estimated from the physician daily logs for patients seen over a two-week period. The number of newly diagnosed and existing GW cases was captured during consultations recorded in the physician daily logs. GW prevalence was estimated using a stratified estimator based on weights calculated to adjust for the difference between the observed number of physicians in each specialty and the (externally) estimated number of physicians in each specialty, at the national level.
The GW prevalence in physician practices was calculated using the number of newly diagnosed or existing GW cases observed, divided by the total number of patients seen during the two-week study period. The prevalence was calculated for overall patients, each physician specialty type, and patient age group and gender. Prevalence by specialty from the study sample was then adjusted to the national level to provide one national prevalence estimate across all specialties.
The prevalence of newly diagnosed and existing cases was estimated using a stratified estimator based on weights calculated to adjust for the difference between the observed and estimated number of physicians in each specialty at the national level. According to estimates by Merck Sharp & Dohme (MSD), a total of 24 000 OB/GYN, 9600 DERM, and 6000 URO practice on the national level in Russia.
Data from the two-week daily logs were also used to estimate GW prevalence for patients aged 18–60 years in four federal districts in Russia (Central, Privolzhskiy, Sibirskiy/Ural, and Northwest). Population estimates of physicians per specialty group (as per MSD) were divided equally among the regions.
Referral patterns and resource use for GW patients were captured through a 30-minute face-to-face physician survey during the study period from January to June 2012. The survey included questions related to resource use, treatment (in-office treatments and procedures, in-office or at-home topical treatments), and follow-up care (office visits, emergency room visits, hospitalizations) for typical GW patients in the practice as part of the standard course of diagnosis. Survey questions were designed to determine patient referral patterns from general practice physicians to specialists as well as between specialists. Referral patterns were assessed using the physician survey, which included the percentage of patients referred by other physicians as well as those consulted directly with OB/GYN, DERM, or URO.
Statistical Analysis. All data analyses and summaries were performed using SAS® Version 9.2. All study outcomes were summarized descriptively. Categorical data were summarized using counts and percentages, and continuous data were summarized using number of observations and mean values.
Prevalence was stratified by patient age group, patient gender, and physician specialty. Number, mean, and 95% CI were reported. The number and percentage of newly diagnosed and existing GW patients were reported by physician specialty type. Recurrent and resistant cases for existing GW patients were specified.
Referral patterns for GW patients were reported descriptively. The number and mean percentage of patients who directly consulted with, or were referred by, each physician specialty type were reported. Health care resource use was reported and compared among physician specialty types.
RESULTS
Prevalence
A total of 103 physicians completed the two-week daily log, including 28 OB/GYN, 40 URO, and 35 DERM. Information was recorded for approximately 15 961 patients, with 1369 GW cases observed in patients aged 18–60 years. When combining the MSD estimates of practicing physicians with the average number of patients reported on the two-week daily log, an overall patient population seen during the two-week daily log period was estimated at 3 811 714 patients for OB/GYN, 842 400 for URO, and 1 617 737 for DERM.
Based on these data, the overall weighted estimated GW prevalence was calculated at 9.2% (95% CI: 8.3%–10.0%). For each specialty group, the unweighted estimated GW prevalence was 9.9% for OB/GYN, 8.3% for URO, and 7.8% for DERM (Table 1).
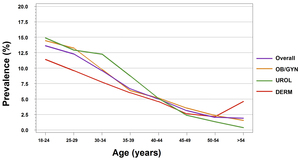
A total of 15 914 patients were reported in the two-week daily logs. Among the 7513 male patients reported in the two-week daily logs, a total of 673 GW cases were reported, resulting in a weighted total observed GW prevalence of 8.8% (95% CI: 7.9%–9.7%). For male patients, the highest prevalence was calculated for those aged 30–34 years (12.1%), followed by 25–29 years (11.4%) and 18–24 years (11.0%) (Figure 1). For each specialty group, the unweighted estimated GW prevalence for male patients was 9.5% for URO and 8.3% for DERM (Figure 2).
Among the 8401 total female patients included in the two-week daily logs, a total of 695 GW cases were reported, resulting in a weighted total observed GW prevalence of 9.3% (95% CI: 8.4%–10.2%). The highest prevalence was calculated for those aged 18–24 years (14.5%), followed by 25–29 years (12.6%) (Figure 1). For each specialty group, the unweighted estimated GW prevalence for female patients was 9.9% for OB/GYN, 5.0% for URO, and 7.3% for DERM (Figure 2).
For male and female patients seeing all physician specialties, the estimated GW prevalence was the highest for patients aged 18–24 years (OB/GYN: 14.4%; URO: 14.9%; DERM: 11.4%). GW prevalence decreased as patient age increased (Figure 2).
Prevalence stratified by region is presented in Table 2. For all patients, the unweighted estimated GW prevalence was the highest in the Sibirskiy/Ural federal district (10.0%), followed by the Northwest (8.9%) and Central federal districts (8.8%). The lowest prevalence was noted in the Privolzhskiy federal district (6.4%).
For male patients, the unweighted estimated GW prevalence was 8.8% in the Central federal district, 8.0% in Privolzhskiy, 14.4% in Sibirskiy/Ural, and 8.9% in the Northwest federal district. For female patients, the unweighted estimated GW prevalence was 8.9% in the Central federal district, 4.9% in Privolzhskiy, 6.9% in Sibirskiy/Ural, and 8.9% in the Northwest federal district.
Overall, 941 newly diagnosed and 407 existing GW cases were identified. Among existing GW cases, 63.1% were recurrent and 34.2% were resistant. The percentage of patients with a resistant form of GW ranged from 19.4% in URO to 46.5% in DERM (Table 3).
For an in-depth view of gender-stratified referral patterns and health care resource use, please see the Supplementary Material, which includes a Supplemental Table and Supplemental Figures.
DISCUSSIONS
This multicenter observational study was conducted in Russia to assess GW prevalence in physician practices and GW-related health care resource use among male and female patients aged 18–60 years.
At the Russian national level, the current study estimated GW prevalence to be 8.8% in male and 9.3% in female patients, which is higher compared to estimates reported in recent literature for other countries.20,21 For instance, the US National Health and Nutrition Examination Survey found that from 1999 through 2004, 5.6% of survey respondents (aged 18–59 years) self-reported a GW diagnosis.22 The percentage was higher for women (7.2%; 95% CI: 6.2%–8.4%) compared to men (4%; 95% CI: 3.2%–5.0%). Although our study did not examine the reasons for the high rate of GW in Russia, several factors could be leading to this high rate. One factor to consider is the knowledge, attitude, and practices surrounding STIs in Russia. A study by Lan et al. observed an association between a high rate of alcohol consumption and an increased risk of STIs, such as HIV, in Russia.23 Other factors could be healthcare accessibility for patients with STIs and inadequate health education on STIs prevention and transmission.
Results from a systematic review of GW incidence and prevalence conducted in four Nordic European countries showed a wide range of prevalence in the self-reported history of GW.24 In surveys of general adult populations, 0.36% (Slovenia, sexually active, aged 18–49 years) to 12.0% (Iceland, aged 18–45 years) of females reported a lifetime history of GW.5 In male populations, 0.27% reported a history of GW in Slovenia from November 2004 to June 2005, and 3.6% to 7.9% reported in Australia, Denmark, the United Kingdom, and the United States.5,22,25–27
For all patients included in the current study, GW prevalence was higher in females than in males (9.3% vs 8.8%). When examining a single gender group, male patients had the highest prevalence at those aged 30–34 years, and female patients at 18–24 years, both followed by patients aged 25–29 years. These results are consistent with data reported in in other countries. In a study conducted in the United States, GW incidence was highest among female patients aged 20–24 years (4.6 cases per 1000) and males aged 25–29 (2.7 cases per 10000).21 Additionally, in an Australian study population, GW incidence peaked in women aged 20–24 years (8.61 cases per 1000) and in men ages 25–29 years (7.40 cases per 1000).28 In Canada, GW prevalence was the highest among females aged 20–24 years (3.88 cases per 1000), whereas prevalence peaked at 25–29 years in men (3.69 cases per 1000).29 Unlike results from the current study, GW prevalence between 1998 and 2006 in Canada was always higher in male than in female patients.
GW prevalence stratified by region showed the highest prevalence to be in the Sibirskiy/Ural federal district and the lowest in Privolzhskiy. The Sibirskiy/Ural federal district includes the cities of Novosibirsk, Chelyabinsk, and Tyumen, while the Privolzhskiy federal district includes Penza, Saransk, Ulyanovsk, Orenburg, and Izhevsk. These are the first available data comparing GW prevalence across regions of Russia.
The most recommended and effective treatment options for GW treatment are podofilox, imiquimod, surgical excision, and cryotherapy.30 In the current study, the treatment pattern reported suggested that podofilox was the most frequently used therapy in male patients. Other topical medications (cryopharma spray, curiosin, cycloferon, epigen, genferon levomekol, panavir, pheresolum, pimafucort, rebif, resorcinol, solcoderm, tricresol, triderm, lactic acid, salicylic acid) were most frequently used to treat female patients. In terms of in-office treatments and procedures, a basic office visit was more frequently conducted by participating physicians. In both male and female patients, a basic office visit was followed by electrosurgery. However, a study that looked at effect of the HPV vaccine in Australia saw a significant yearly decrease in the diagnosis and management rate of GW among women of vaccine-eligible age, indicating a decrease in the development of GW among this population.31
Although there are limited data on GW in Russia, the burden of cervical cancer in the country indicates alarming rates in certain regions. In fact, incidence and mortality from cervical cancer are substantially higher in Eastern than in Western European countries mainly due to the lack of effective screening programs.18,32
In Russia, more than 6000 women succumb to cervical cancer annually, accounting for approximately 4.6% of all cancer-related deaths among Russian women.18 No organized screening programs exist in Russia, and cervical cancer prevention is based on opportunistic screening with low coverage. Screening is also poorly standardized without quality-assured cytology and colposcopy.18
The current study has several limitations. The national prevalence estimates were based on the physician population available from medical societies and may not include all practicing physicians in Russia. In addition, estimates and results in this study are representative of the urban population of Russia.
GW patients who did not seek health care were not included, which may underestimate the true prevalence in Russia since weighting was applied.
Potential bias related to the information source, based on a physician survey for the estimation of health care resource use, may exist since bias in recall estimations by physicians can be difficult to control for.
CONCLUSIONS
GW is a significant public health concern. The aim of this study was to estimate the burden of GW as well as the GW-related health care resource use for male and female patients aged 18–60 years in Russia. The overall GW prevalence in Russia was estimated at 9.2%, with higher prevalence in female compared to male patients. GW prevalence was highest in female patients aged 18–24 and male patients aged 30–34 years.
OB/GYN and URO practices saw a high prevalence of GW patients. Visual examination was the most common diagnostic tool used by all physician specialists for both male and female patients. Despite its limitations, this study provides a GW prevalence estimate in Russia not previously available. In addition, we recommend further studies to evaluate the factors contributing to the high prevalence of GW in Russia.
DECLARATIONS
Ethics Approval and Consent to Participate
Ethical approval was obtained from an accredited external IRB for those sites not covered by their own internal IRB. The ethical conduct of this study was performed in accordance with the Declaration of Helsinki and the principles of Good Clinical Practices.
Consent for Publication
Not applicable.
Availability of Data and Material
The data analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
V.N. Prilepskaya and M. Gomberg have no conflicts to disclose.
S. Kothari and A. Kulkarni are employees of Merck & Co.
K. Yee was a paid contractor for Merck & Co. at the time of the study and was an employee of Cubist Pharmaceuticals DEC2014-JUL2015; it was acquired by Merck & Co. in January 2015.
S.M. Garland received grants from the Commonwealth Department of Health for HPV, Merck & Co., and Glaxo Smith Kline to perform phase 3 clinical vaccine trials: Merck to evaluate HPV in RRP post vaccination program; CSL for HPV in cervical cancer study, and VCA for a study on the effectiveness of a public health HPV vaccine study, and a study on the associations of early onset cancers. Merck & Co. paid for travel and accommodation to present at HPV Advisory board meetings.
A.R. Giuliano is a member of Merck & Co, Inc. advisory boards. Her institution has received grants and contracts to support HPV-related research.
Funding
This study was funded by Merck & Co., Inc.
Authors’ Contributions
V.N. Prilepskaya, M. Gomberg, S. Kothari, K. Yee, A. Kulkarni, S.M. Garland, and A.R. Giuliano conceived and designed the experiments as well as contributed to the writing of this manuscript.
V.N. Prilepskaya, M. Gomberg, S. Kothari, and A. Kulkarni performed the experiments for this manuscript.
V.N. Prilepskaya and M. Gomberg analyzed the data for this manuscript.
V.N. Prilepskaya and M. Gomberg contributed reagents/materials/analysis tools for this manuscript.