INTRODUCTION
Glycogen storage disease type Ia (GSDIa) is a rare inherited disease due to an inborn error of carbohydrate metabolism caused by variants in the G6PC gene.1 G6PC encodes glucose-6-phosphatase (G6Pase),1 an enzyme critical for glucose homeostasis. GSDIa causes a deficiency of G6Pase, minimizing the ability to release or generate glucose from the liver. Patients are at risk for severe hypoglycemia and metabolic abnormalities (eg, excess glycogen storage; production of lactic acid, uric acid, alanine, and triglycerides).2–4 Early diagnosis and treatment are essential for minimizing long-term complications.
There are no approved pharmacologic therapies targeting the underlying cause of GSDIa. Current management involves a regimented, medically prescribed diet typically requiring uncooked cornstarch every 3 to 6 hours, with or without a high-amylopectin waxy maize starch (Glycosade®), scheduled meals high in complex carbohydrates, and avoidance of nonutilizable sugars and fasting. Cornstarch and Glycosade® are digested slowly to help stabilize blood glucose when taken as scheduled.5 Although uncooked cornstarch has been the mainstay of GSDIa glucose management for decades, it requires lifelong patient and family commitment, negatively impacts quality of life, and can lead to wide fluctuations in blood glucose levels, suboptimal metabolic control, and chronic complications.6,7 Missing a single dose may cause severe hypoglycemia with lactic acidosis and even death.
There is a substantial humanistic burden associated with GSDIa. Patients experience hypoglycemic events, despite best efforts toward condition management.8 Adherence to a strict treatment regimen is challenging. Cornstarch can be unpalatable and cause gastrointestinal symptoms. There is constant fear that missing or delaying a single dose could result in a severe hypoglycemic event. Frequent cornstarch consumption interrupts daily activities and patients and caregivers describe a variety of negative physical, emotional, and psychosocial consequences.8–12
DTX401 (pariglasgene brecaparvovec) is an investigational adeno-associated virus serotype 8 vector (AAV8)–based gene therapy designed to deliver the human wild-type G6PC transgene to hepatocytes and restore endogenous glucose production. In a phase 1/2, open-label, safety and dose-finding study of DTX401 in adults with GSDIa (NCT03517085), participants in all cohorts showed significant cornstarch reductions from baseline to Week 52. DTX401 treatment was well-tolerated and had an acceptable and manageable safety profile.13
As part of the DTX401 phase 1/2 study protocol,13 qualitative interviews were conducted with trial participants at study Weeks 24, 52, and 104 to explore their clinical trial experience, any changes in GSDIa-specific health-related quality of life (HRQoL) following DTX401 treatment, and perspectives on gene therapy in relation to existing standard-of-care treatment.
METHODS
Sample
Twelve adults (≥18 years) with GSDIa recruited from clinical sites in the United States (US), Canada, the Netherlands, and Spain participated in A Phase 1/2, Open-Label Safety and Dose-Finding Study of Adeno-Associated Virus (AAV) Serotype 8 (AAV8)-Mediated Gene Transfer of Glucose-6-Phosphatase (G6Pase) in Adults with Glycogen Storage Disease Type Ia (GSDIa) (NCT03517085, NCT03970278).13 While qualitative interviews were planned after the start of the trial, they were intended to be conducted as part of the trial protocol (ie, embedded interviews). The interview inclusion criteria included individuals participating in the phase 1/2 trial and there were no exclusion criteria. Because the interviews were planned as part of a protocol amendment after the trial had already started, only a subset of trial participants were able to be recruited for interviews; this included only those who had not yet passed their Week 24 or Week 52 study visit by the time ethics approval was received. Clinical site staff contacted trial participants to identify and schedule possible interview dates as close as possible to their Week 24, Week 52, and Week 104 study visits. All direct communication with participants was conducted by the clinical site staff. Lumanity (formerly known as Endpoint Outcomes) researchers provided a conference dial-in to clinical sites for participants to use at the time of their scheduled interview.
Materials
Semistructured interview guides (key questions shown in Supplementary Table S1) were used to explore patient perspectives on GSDIa, gene therapy, exogenous glucose intake, diet, and treatment satisfaction. Specifically, the Week 24/Week 52 and Week 104 interview guides included topics, questions, and probes designed to explore the effect gene therapy had on symptoms and impacts of GSDIa, the effect gene therapy had on diet and treatment (eg, cornstarch intake), and satisfaction with treatment. Week 52 and Week 104 interviews followed up on changes to cornstarch intake based on responses provided during the prior interviews.
Procedure
Following ethics approval, 30-minute telephone interviews were conducted with each participant by Lumanity researchers who have expertise in patient-centered outcomes research via web-based teleconference at or around Weeks 24, 52, and 104. Interviews were audio-recorded with participant consent; and data were transcribed, anonymized, coded using version 8.0 or higher of ATLAS.ti, and analyzed.14,15 Each transcript was coded (ie, codes were applied to specific text within each transcript) by one of four Lumanity researchers, and a research supervisor addressed any discrepancies or questions. All coders received comprehensive instruction in established qualitative research methodologies, including grounded theory and the constant comparative method. In addition, each coder completed specialized training in coding procedures tailored to concept elicitation and trial interviews, as well as training in the use of ATLAS.ti, the qualitative analysis software employed during the project.
The coding process (codebook sample shown in Supplementary Table S2) was guided by established qualitative research methods, including grounded theory and constant comparative method. Specific grounded theory methods applied to this research included collecting and analyzing data in parallel (ie, initiating coding of transcripts while interviews are ongoing), letting the coding scheme be dictated by the data and not preconceived notions, constantly comparing and contrasting concepts to inform relationships among the data (ie, constant comparative method), and using memos within individual transcripts as necessary to explain findings and inform the next step of analysis (ie, aggregating transcripts and harmonizing codes).14,15 Frequencies of concepts were reported, with accompanying exemplary quotes. Thematic saturation of concept was not evaluated; data collected from all available trial participants was reported.
RESULTS
Sample Characteristics
While the trial sample included 12 participants, trial interviews were implemented after the trial had already started. No baseline interviews were conducted. Also, some participants (Cohort 1, n = 3; Cohort 2, n = 1) had already passed their Week 24 or Week 52 study visit; thus, interviews were not conducted with those participants. One Cohort 2 participant was interviewed but not recorded due to ethics restrictions. Thus, the analysis sample included 7 patients (n = 5 at Week 24, n = 7 at Weeks 52 and 104) interviewed between October 2019 and November 2022. The sample mean age at baseline was 27.4 years; 43% were female (Table 1).
Changes in Signs/Symptoms, Functional Impacts, and HRQoL
Across interview timepoints, most participants (86%, n = 6/7) primarily reported positive and meaningful changes in GSDIa following DTX401 treatment, although 1 participant (14%) reported no change in GSDIa. Three (43%, n = 3/7) reported no negative changes in HRQoL following DTX401 treatment, and 4 (57%, n = 4/7) reported at least one negative change, primarily at Week 104. Reduction in cornstarch intake was identified as a top improvement at both Weeks 52 and 104.
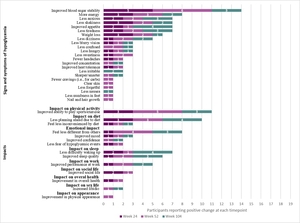
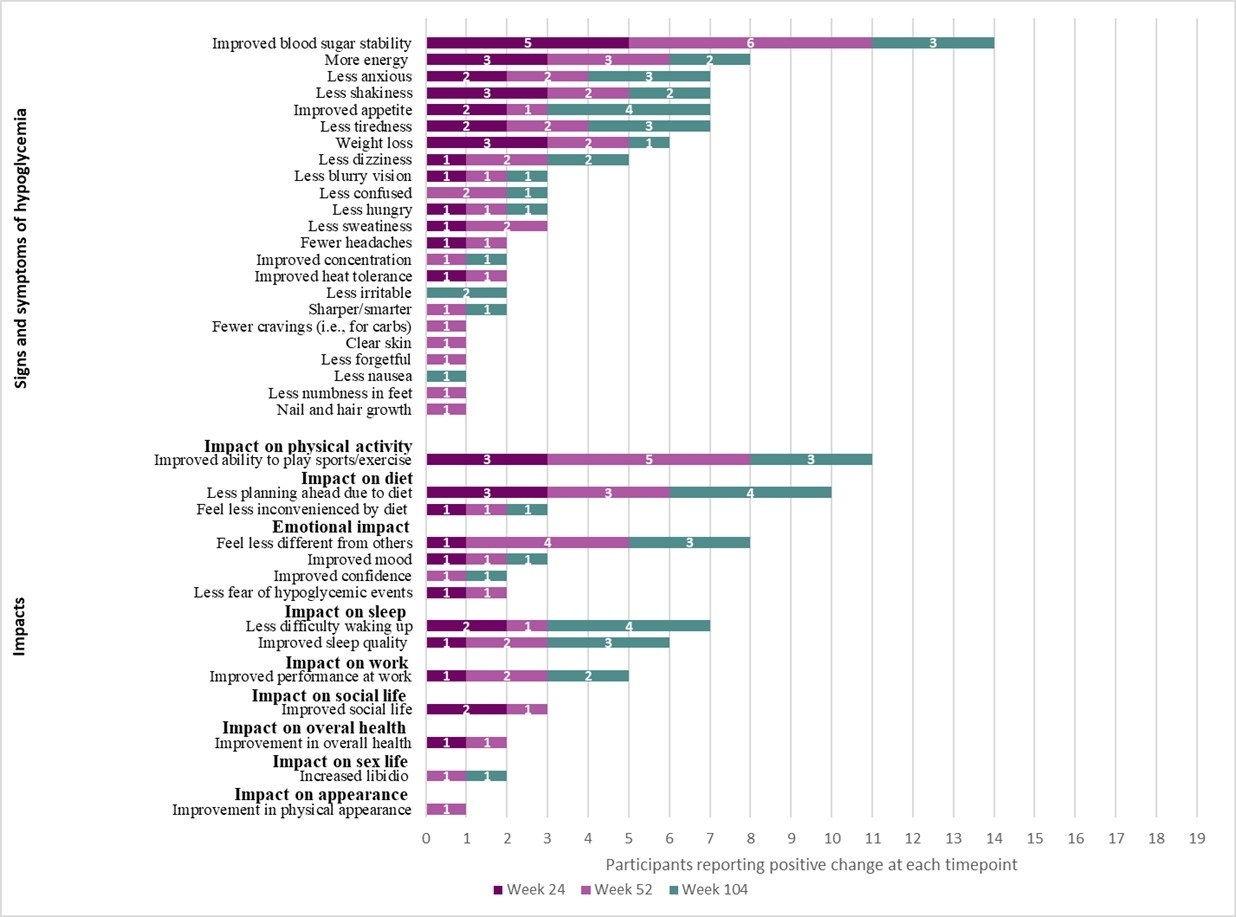
Signs/symptoms: Across interview timepoints, participants most commonly reported improved blood sugar stability, more energy, less anxiety, less shakiness, improved appetite, less tiredness, weight loss, and less dizziness (Figure 1).
During Week 24 interviews, participants most commonly reported improved blood sugar stability (100%, n = 5/5), less shakiness (60%, n = 3/5), more energy (60%, n = 3/5), and weight loss (60%, n = 3/5). Participants described improved blood sugar stability both in terms of no longer having lows and having stable blood sugar.
During Week 52 interviews, participants most commonly reported improved blood sugar stability (86%, n = 6/7) and more energy (43%, n = 3/7). A few new changes were reported at Week 52 by participants that were not reported during Week 24. Two participants (29%) described less confusion; and clear skin, less forgetful, better concentration, mentally sharper, increased confidence, improved appearance of skin, nails, and hair, improved libido, reduced cravings, and less numbness in feet were each mentioned by 14% (n=1/7) of participants.
During Week 104 interviews, participants commonly reported improved appetite (57%, n = 4/7), blood sugar stability (43%, n = 3/7), less anxiety (43%, n = 3/7), and less tiredness (43%, n = 3/7). New changes reported during Week 104 included less irritability (29%, n = 2/7) and less nausea (14%, n = 1/7).
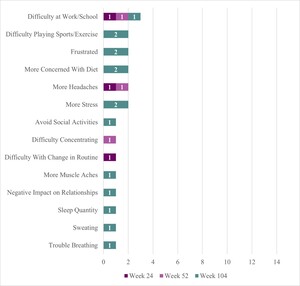
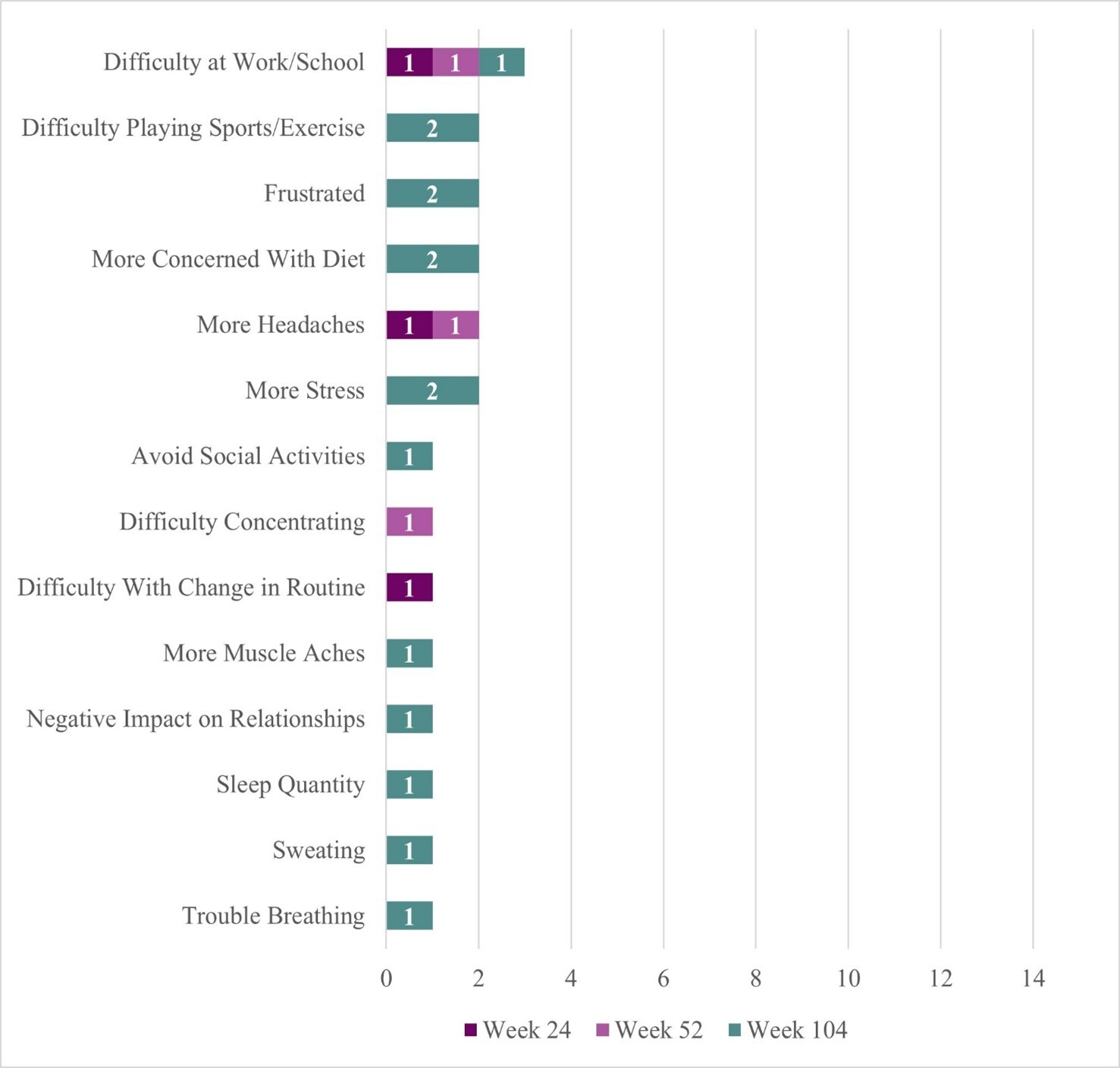
Two participants reported some negative sign/symptom changes (Figure 2). One participant reported headache at Week 24, headache and difficulty concentrating at Week 52, and frustration, stress, sweating, heavier breathing when blood sugar is high, and muscle ache (although no longer reporting frequent headaches or difficulty concentrating) at Week 104. This participant described life-changing benefits from DTX401 treatment (“it’s like a new life…a new beginning”) but shared that condition management can be stressful because “there is not an exact formula on how to handle [their] health now.” They reported that they had improvements in concentration and focus at Week 104 because they no longer needed to constantly “look at the clock” to manage their GSDIa. This same participant attributed the muscle ache to gout; explained that sweating was due to greater sensitivity to heat at Week 104 rather than instances of low blood sugar; and that they breathe heavier when their blood sugar levels are high (which they do not like experiencing, but interpreted positively as a signal that they need to take action to lower their blood sugar level).
The second participant reported no negative symptoms at Week 24 and Week 52 but described experiencing frustration and stress at Week 104. This participant explained that while their cornstarch intake was lower, they could “last longer” until the next dose, and eat more of their desired foods (eg, spaghetti), they experienced some frustration and stress due to instances of low blood sugar (though not as severe/low as prior to treatment) and changes to their diet management, requiring them to adapt their lifestyle at Week 104.
Table 2A presents sample quotes from interview participants describing changes in signs/symptoms following DTX401 treatment.
Functional impacts: Across interview timepoints, participants most notably reported improved physical activity (eg, increased ability to play sports/exercise), diet (eg, less planning ahead for diet), emotional function (eg, feeling less different from others), and sleep (eg, less difficulty waking up, better sleep quality) (Figure 1).
During Week 24 interviews, participants reported improved ability to play sports/exercise (60%, n = 3/5) and less planning ahead due to diet (60%, n = 3/5). Participants described improved physical stamina, increased energy that enabled improved physical function, greater flexibility in diet, and less fear of fluctuating blood sugar levels, reducing worry and improving participation in daily life routines.
During Week 52 interviews, participants most commonly reported improved ability to play sports/exercise (71%, n = 5/7), feeling less different than others (57%, n = 4/7), and less planning ahead due to diet (43%, n = 3/7).
During Week 104 interviews, participants reported improved appetite, less difficulty waking up, and less planning ahead due to diet, which were each reported by 4 participants (57%, n = 4/7). Further, some participants described that they were able to have more flexibility in their diet, felt more refreshed upon awakening, and experienced longer fasting intervals.
Four participants reported some negative changes in functional impact (Figure 2). The same participant who reported headache and difficulty concentrating symptoms at earlier trial timepoints reported more difficulty at work/school at Week 24 (20%, n = 1/5) which persisted for this same participant at Weeks 52 and 104, and attributed this difficulty to changes in their blood sugar levels and the “fine tuning” required to “fix” them, noting that they need to stop what they are doing to address them (eg, “snack consistently”). This same participant reported difficulty with change in routine at Week 24 (attributed to the need to manage a changing diet regimen following gene therapy), and more concern with diet, negative impact on relationship with family and friends (more focus on condition management and less time for relationships), and sleep quantity (although sleep quality improved) at Week 104. One participant described more concern with their diet at Week 104. This is the same participant who reported overall positive outcomes from treatment yet increased frustration and stress symptoms at Week 104, attributable to adaptations needed for diet management following treatment. Finally, 2 participants reported difficulty in sports/exercise at Week 104 despite improvement reported in this concept at Weeks 24 and 52. At Week 104, both reported that their ability to play sports/exercise was about the same as it was prior to receiving treatment. One participant posited that the improvement experienced at earlier timepoints may have been affected by other medicines (eg, corticosteroids).
Table 2B presents sample quotes from interview participants describing changes in their functional impacts following DTX401 treatment.
Overall HRQoL: Most participants described overall HRQoL improvements at Week 24 (80%, n = 4/5), Week 52 (86%, n = 6/7), and Week 104 (71%, n = 5/7); 1 participant reported no improvement in overall HRQoL across timepoints (Table 2C).
Top improvements: Across interview timepoints, the concepts most frequently identified as top improvements included reduction in cornstarch intake, improved energy levels, and improved blood sugar stability (Table 2D).
Patient Perspectives on Study Treatment
Most participants with evaluable data at Week 24 (75%, n = 3/4), Week 52 (67%, n = 4/6), and Week 104 (57%, n = 4/7) reported they would still want gene therapy even if they had to adhere to a restricted diet (Table 2E). Similarly, most participants at Week 24 (100%, n = 5 of 5), Week 52 (100%, n = 7/7), and Week 104 (71%, n = 5/7) said they would still want gene therapy treatment even if they had to continue cornstarch (Table 2F).
Perspectives on taking cornstarch: Four participants each reported liking one aspect of cornstarch at Weeks 24, 52, and 104, with reasons including not having to eat as much when taking cornstarch, taking before bedtime helped them sleep through the night, helping them feel stable/safe, and helping them fix a problem with their blood sugar (Table 2G). Three participants, across all interview timepoints, reported not liking any aspect of taking cornstarch. Participants commonly described disliking the inconvenience of the regimen, bloating, high calorie and carbohydrate count, and bad taste (Table 2G).
Perspectives on taking DTX401 gene therapy: When asked what they liked about taking DTX401, across timepoints, participants most commonly described the efficiency of the therapy as a one-time painless infusion, the potential for improvement, reduced need for cornstarch, and greater freedom in life (Table 2H).
When asked what they disliked about taking DTX401 study treatment, across timepoints, participants most commonly described that they disliked taking steroids, the trial visit frequency, or that there was nothing they disliked about DTX401 gene therapy (Table 2H).
Treatment Expectations and Satisfaction with Treatment
Treatment expectations: The most commonly reported treatment expectations across timepoints included reduction or elimination of cornstarch, improved blood sugar stability, and more flexible diet. Other expectations included improved ability to play sports/exercise, weight loss, more energy, and improved overall health (Table 2I).
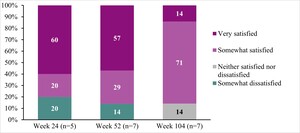
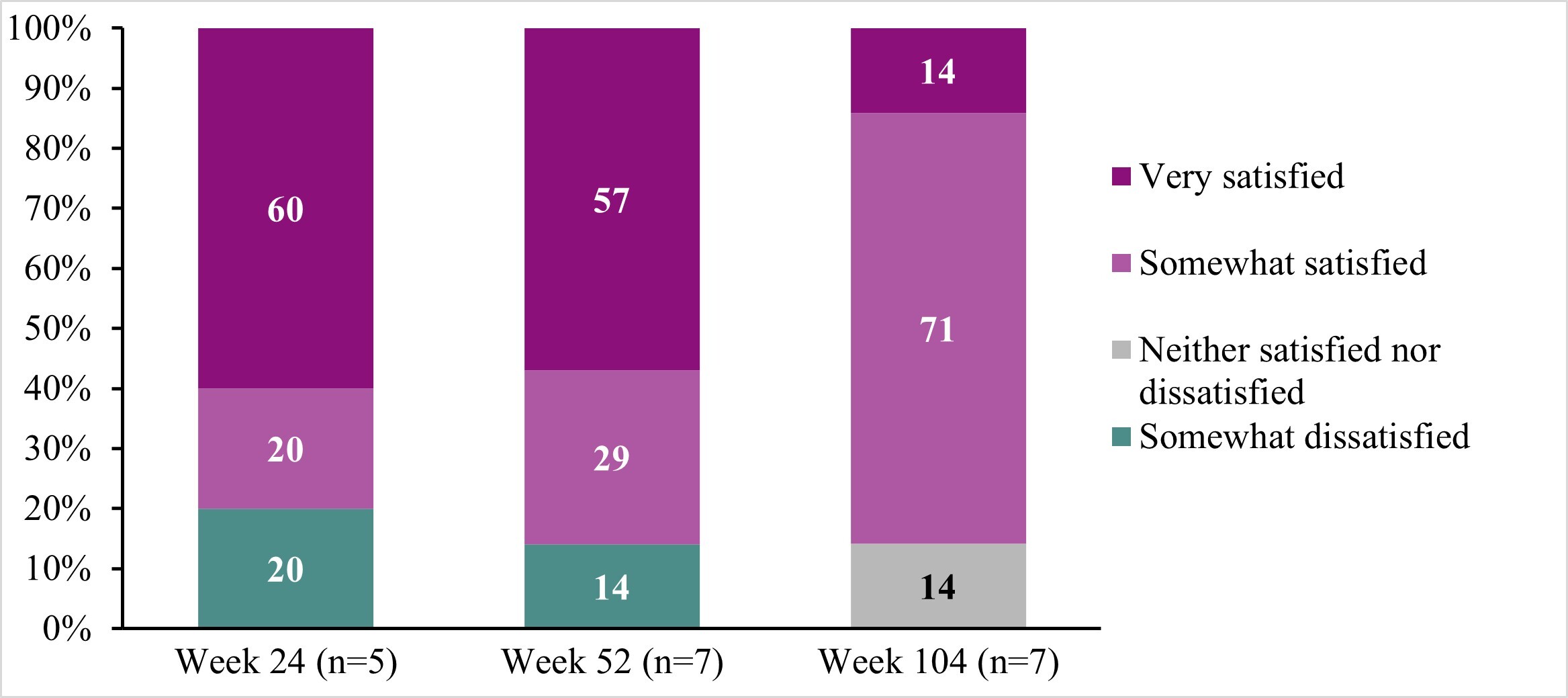
Treatment satisfaction: While participant reports of satisfaction fluctuated across interview timepoints, most were somewhat satisfied or very satisfied with DTX401 gene therapy at Weeks 24 (80%), 52 (86%), and 104 (86%), and no participants reported being very dissatisfied at any interview timepoint (Figure 3; Table 2J).
DISCUSSION
During trial interviews, most DTX401-treated participants reported substantial reduction in cornstarch intake, aligning with reported trial results.13 Interview participants perceived corresponding improvements in HRQoL, including improved symptoms, physical function, diet management, emotional function, self-perception, social function, sleep quality, work performance, and overall health. Across interview timepoints, participants most consistently reported improved blood sugar stability, improved ability to play sports/exercise, less planning ahead due to diet, less shakiness, more energy, weight loss, and improved sleep quality as positive changes. Top improvements reported by participants across interview timepoints included reduction in cornstarch intake, improved energy levels, and improved blood sugar stability. Overall, few negative changes were reported, most of which were at Week 104. Findings suggest that DTX401 helps to address aspects of the condition and its standard of care treatment that patients have identified as burdensome.8,16–18
While there were some mixed results regarding whether trial expectations were fully met, most participants preferred gene therapy over standard-of-care treatment, most liked the efficacy of gene therapy as a one-time infusion, and most indicated that they would want gene therapy even if they had to continue cornstarch and even if they still had diet restrictions.
Most reported satisfaction with treatment across interview timepoints, although some fluctuations were reported. No participants reported being very dissatisfied with treatment at any of the timepoints. Trial procedures and tests that may have influenced satisfaction ratings could be addressed in future research through patient education to clarify trial requirements.
The following opportunities for improvement were identified: (1) preparation and patient support for the dietary changes required by transitioning from cornstarch as a major source of calories to a more balanced diet and (2) review of glycemic control data with patients to facilitate shared decision-making on nutritional adjustments and reduce fear of hypoglycemia when cornstarch doses are reduced.
This interview study had several limitations. One major limitation was a very small sample size and a demographically homogeneous sample (7/12 participants, all White), driven by sampling from a small, phase 1/2 ultra-rare disease trial population. An important strength of this interview study is the longitudinal design, which captures the participant experience over multiple trial timepoints. However, because the interview research started after the phase 1/2 trial was underway, only a subset of trial participants (n = 7/12) were interviewed, and no baseline interviews were possible. Baseline interviews to capture burden prior to trial treatment could help to limit potential bias when assessing the experience of change over time at the participant level. Quantitative results from the phase 1/2 trial have been published,13 and this interview research yields important insights into the patient experience of DTX401 treatment to support the interpretation of quantitative outcomes. Future research of this nature could be improved using a mixed-methods approach that integrates the qualitative and quantitative analysis. Another consideration is that this research describes interview participants’ perception of their trial experience without formal testing of relationships among variables. While four trained researchers with expertise in patient interview research coded transcripts over the course of this lengthy study and efforts were made to ensure consistency in the coding approach (eg, any questions/uncertainties/discrepancies reviewed by a supervising researcher), the coding process was limited in that only one interview researcher coded each transcript and coding confirmation (and interrater reliability) was not assessed as part of the process. Additionally, interview conduct was contingent on trial visits proceeding as intended during the COVID-19 pandemic. Finally, a longer interview duration would have provided more time for additional in-depth probing questions.
CONCLUSION
Most participants interviewed during an open-label, phase 1/2 dose-escalation trial (NCT03517085) evaluating the safety and efficacy of DTX401 in adults at least 18 years of age with GSDIa described positive experiences, including reduced treatment burden, improved HRQoL, and satisfaction with treatment. Results from an open-label trial may be exploratory, be subject to potential bias, and have limited generalizability. Nevertheless, these qualitative trial interviews provide important insights into the patient-reported experience of trial participation, potential for this investigative treatment, and support for the interpretation of quantitative trial outcomes. Results suggest that to optimize outcomes and patient experience with gene therapy for GSDIa, anticipatory guidance on dietary changes and the need for close monitoring during implementation of these changes should be provided. Findings from the ongoing phase 3 randomized, double-blind, placebo-controlled study of investigative DTX401 (NCT05139316) will further inform the GSDIa patient experience of treatment.
Acknowledgments
The authors thank the patients, principal investigators, and clinical site staff for their important contributions to this research; Maia Bowker, medical writer at Ultragenyx, for her help in writing and editing this manuscript; Michelle Kelly of Ultragenyx for her review and editorial support; and Ashley O’Mara of Ultragenyx for her contributions to the design of the work. We also acknowledge the research contributions of Betsy Malkus while previously employed at Ultragenyx, and Chris Evans, who is currently employed at Lumanity.
Funding
This study was funded by Ultragenyx Pharmaceutical, Inc.
Ethics Approval and Patient Consent Statement
The protocol, including embedded trial interviews, was approved by the institutional review board at each participating center. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients prior to participation in the study.
Meeting Presentation
Components of this research were summarized in a poster presentation at the Society for Inherited Metabolic Disorders (SIMD) 45th Annual Meeting, Charlotte, North Carolina, April 14-17, 2024.