INTRODUCTION
Colorectal cancer (CRC) is the second most common cause of cancer-related mortality in western countries. The 5-year relative overall survival rate of patients with metastatic colorectal cancer (mCRC) is approximately 14%. In this context, the disease prognosis markedly worsened by molecular changes in the Kirsten rat sarcoma virus (KRAS), the neuroblastoma RAS viral oncogene homolog (NRAS), and the B-Raf proto-oncogene (BRAF).1 RAS mutations are found in approximately half the patients diagnosed with mCRC. These mutations are also linked to poor prognosis and a lack of responsiveness to anti-epidermal growth factor receptor (EGFR).2
According to the World Health Organization 2020 GLOBOCAN, the highest number of new cancer cases in the Kingdom of Saudi Arabia (KSA) in both sexes and all ages was CRC. In Saudi males CRC was the most prevalent cancer, with 19.3% of new cases in 2020, while it was the third most prevalent cancer type in females with 9.2% of new cases in 2020, after breast and thyroid cancers. According to GLOBOCAN 2020, the death rate from CRC in Saudi Arabia was 8.3%.3
The incidence of CRC in KSA has increased over the last 4 decades.4 The median ages at CRC presentation in Saudi women and men are 55 and 60 years, respectively.5 The 5-year survival rate of CRC among the Saudi population is between 44% and 52%, while it is higher in the United States, where the rates reached 60%.4
In Saudi patients with CRC, late diagnosis and up to 50% of distal and regional metastases have been noted, despite the country’s well-established healthcare system. The Saudi public reported little knowledge about CRC, which could contribute to late presentation.6 In addition, young Saudi patients with CRC presented with more advanced stages and poorly differentiated cancers than older Saudi patients with CRC.7
The evaluation of genetic alterations is a crucial component of customized cancer care in the modern era. Targeting wild-type RAS in metastatic colon cancer is an example of how clinical awareness of some of these mutations and their prognostic and predictive potential have transformed the treatment of several malignancies in recent years, improving patient care and prognosis.8 Patients with KRAS-mutated CRC have a higher tendency for lung metastases than liver metastases, which signifies the need for more extensive chest imaging for effective presentation.4 In the Saudi population, the frequency of KRAS mutations is 42.2%, similar to the frequency of KRAS mutations in the Arab population (44.4%) but greater than the KRAS frequencies in Western countries (30%-37%).9–12
Cetuximab (CET) and panitumumab (PAN) are monoclonal antibodies that target EGFR and are used globally to treat patients with mCRC. These biological agents can be used for CRC alone or in combination with chemotherapy (CT) in patients with RAS mutations.13
The two biological agents have the same mechanism of action and pharmacological activity (being active for the first line to more advanced lines of therapy) and same administration schedule based on the new dose regimen under FDA approval (biweekly administration for CET); however, they differ in their chemical structure, and toxicity profiles.13
The choice of proper anti-EGFR therapy for CRC depends on several factors, including the adverse events (AEs) of each biological agent, which will in turn influence the management costs of each agent, patients’ quality of life, and adherence to treatment.14
Objective
The objective of this economic study is to support healthcare decision makers by comparing the costs associated with AEs for the biweekly regimens of both CET and PAN in previously untreated RAS wild-type mCRC in the KSA over a 1-year time horizon.
The tool was developed from two perspectives: the Saudi payers’ perspective and the Saudi societal perspective. This tool allows users to compare the total cost of the AEs associated with CET and PAN.
This is the first economic tool to evaluate AE management costs for mCRC treatments within the Saudi healthcare setting.
METHODS
Study Design
To build our study, we performed a systematic literature review across PubMed, Web of Science, Cochrane, and Scopus to extract all relevant clinical and economic evaluation studies on CET- and PAN-related AEs and their management in the treatment of mCRC using these keywords: “safety,” “cetuximab,” “panitumumab,” “adverse events rate,” “adverse drug reactions,” “wild type RAS,” “metastatic colorectal cancer,” “first line,” “Phase III,” “clinical trial,” “randomized study,” “meta-analysis,” “cost,” and “systematic review.” Thirty studies were identified and 25 studies were excluded after reading the abstract due to different comparators, different subgroups, different lines of therapy, phase II studies, and different outcomes.
Local practices and assumptions of the model were obtained and validated by interviewing local Saudi experts using a structured questionnaire. The clinical inputs for our model were obtained from the literature. The AEs were managed according to Saudi healthcare settings.
Model Structure
This economic model estimates the AE management cost model to calculate the mean number of AE management costs associated with first-line treatment with CET vs PAN regimens for the treatment of mCRC with RAS mutations. The model is a global model designed in Microsoft Excel®, and costs are captured from KSA healthcare settings.
The outcomes of this model were as follows: mean number of AEs associated with CET vs PAN regimens per patient, estimation of the costs associated with AE management per patient, and population level of these regimens. All costs in the model were represented by the Saudi riyal (SAR) and as per the Saudi 2024 payer price lists. We provide results from both the Saudi payer and societal perspectives.
Target Population
The eligible patient population was determined from the published literature and as per the feedback of the interviewed panel of KSA experts. The Delphi panel comprised 8 experts: 1 health economist, 3 payers, and 4 oncology experts from Aseer Central Hospital, King Fahad Medical City, National Guard Health Affairs, Riyadh, KSA. We collected insights from experts through 4 rounds of virtual meetings by using the quasi-Delphi panel approach. The experts’ insights included the current local clinical practice and treatment patterns within the Saudi healthcare settings. The collected experts’ responses were subsequently analyzed and reviewed by all authors to confirm their accuracy and relevance to have consensus. In addition, the clinical oncologists were asked to validate the way safety data are presented in the regulatory label, based on their experience in treating patients with mCRC, to provide their perspective on the occurrence and severity of AEs.
The number of eligible patients for CET treatment was also estimated to determine the total cost impact of AE management in the KSA. This considers whether a patient is eligible for anti-EGFR therapy but not eligible for CET. The target population within our model was calculated as shown in Table 1.
The model’s target population was based on the KSA population (36 408 820) and defined as those with wild-type RAS mCRC treated with anti-EGFR as first-line therapy. The age-standardized ratio incidence of CRC in Saudi Arabia (13.9/100 000) and the percentage of patients who developed metastasis (50%) were derived from published literature.4,15,16 The prevalence of wild-type RAS mutations (50%) was derived from the available literature.17 owing to the lack of local data in the registries regarding the actual prevalence of this type of mutation. The eligible adherent population receiving CET or PAN was 36% based on the local market share data. The target eligible population was 455 patients.
Clinical Inputs
Clinical data on the frequencies of common and very common AEs and their severity were obtained from summaries of product characteristics (SmPCs) and a meta-analysis, respectively.13,18,19 Categorical values were transformed into numerical values using the mean frequencies for each AE. The AE management protocols, clinical model inputs, and assumptions were validated by clinical oncologists affiliated with the National Guard Health Affairs and the Ministry of Health in the KSA.
This study’s calculator only considered the common and very common AEs stated in the SmPC of CET and PAN by the European Medicines Agency.18,19 Those common and very common ADRs usually occurred in Saudi population as per our validation from the local experts. Summaries of product characteristics are publicly available regulatory documents approved by the European Medicines Agency as part of marketing authorization and are used as the basis of information for health professionals on how to use medicines. The European Medicines Agency considers the following frequency terminology definitions:
-
Very common (≥1/10)
-
Common (≥1/100 to <1/10)
The probabilities of severe AEs used in our model were extracted from a 2018 meta-analysis study that included a total of 38 studies to build the analysis results.13 In addition, the missing probabilities of severe AEs were assumed to be the average of severe AEs reported in the 2018 meta-analysis study.13 The probability of AEs was illustrated in a 2018 meta-analysis. The number of AEs for all-grade AEs and severe AEs (Grade 3-4 AEs) is shown in Table 2 and Supplementary Table S1, respectively. The details of the AE management protocols are summarized in Supplementary Table S2. We assumed that each patient with severe AE was hospitalized based on local clinical practice, while patients with grade 1 and 2 A/Es were treated as outpatients.
Costs
All the unit costs used in the model are listed in Supplementary Table S3. The model included all resources used to manage AEs associated with the treatment of previously untreated mCRC patients with wild-type RAS in Saudi healthcare settings. The unit costs were obtained from a Saudi 2024 database, with health unit costs from the National Guard Health Affairs and Ministry of Health records and were included in the SAR. The total costs of AEs were measured by multiplying the number of targeted patients by the adverse events probabilities multiplied by the unit costs of each event. All output costs transferred to international dollars (Intl$) by using purchasing power parity conversion rate (1.95) sourced from the World Bank.
The model demonstrates the results in terms of the base costs. From a societal analysis perspective, we included indirect costs caused by the loss of productivity owing to the management of AEs. We assumed that each hospital’s day care resulted in productivity day loss for the patient. The Saudi patient average wage per day was estimated using the Saudi gross domestic product published by the World Bank for 2022.20
Sensitivity Analyses
This model allows the user to run a one-way sensitivity analysis to estimate the range of total costs of AEs associated with CET and PAN. A sensitivity analysis was conducted using the upper and lower limits of the AE frequency definitions (ie, very common, >1/10; common, >1/100 to <1/10). The model also demonstrates the results in terms of the minimum and base costs. The minimum cost was assumed to be the base cost divided by length of stay to obtain the average daily cost of treating AEs. A one-way sensitivity analysis was conducted to test the lower-bound and upper-bound unit costs of AEs by using a ±20% range as a plausible wide range to address any uncertainty in the model. Probabilistic sensitivity analysis was conducted to evaluate the simultaneous variation on 1000 iterations of costs and ADR rates using gamma distribution and beta distribution, respectively. Therefore, each distribution used should be relied on the type of variable data.
RESULTS
Base Case
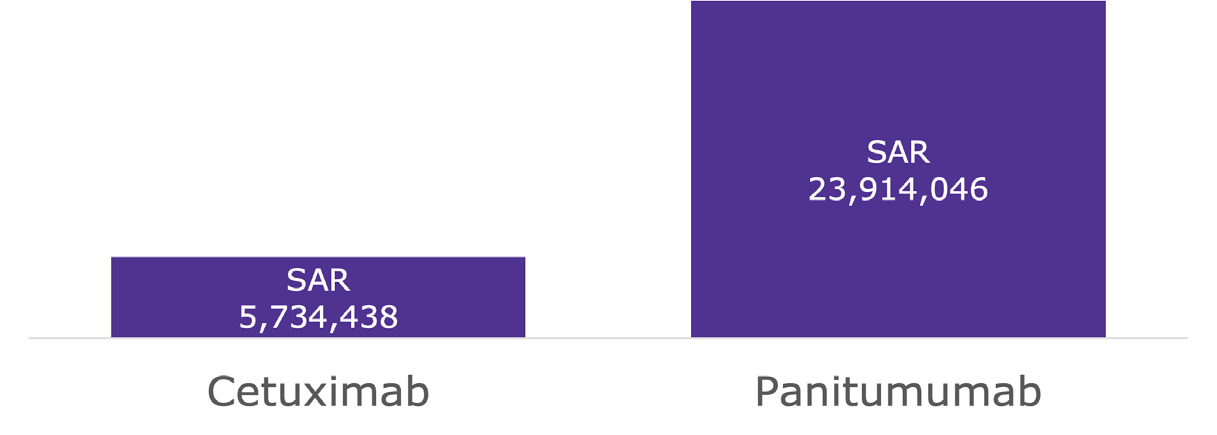
Within the Saudi payer perspective model presented in Table 3 and Supplementary Figure S1, treatment with CET + CT vs PAN + CT resulted in average population cost savings of SAR 9 246 133 (Intl$4 741 606) and per-patient cost savings of SAR 20 321 (Intl$10 421) for severe AEs, while resulting in average population cost savings of SAR 16 039 427 (Intl$8225 347) and per-patient cost savings of SAR 35 251 (Intl$18 077) for all grade AEs. Moreover, within the Saudi societal perspective model (presented in Table 4 and Figure 1), treatment with CET + CT vs PAN + CT resulted in average population cost savings of SAR 11 386 314 (Intl$5 839 135) and per-patient cost savings of SAR 25 025 (Intl$12 833) for severe AEs, while resulting in average population cost savings of SAR 18 179 608 (Intl$9 322 875) and per-patient cost savings of SAR 39 955 (Intl$20 489) for all-grade AEs.
Sensitivity Analysis
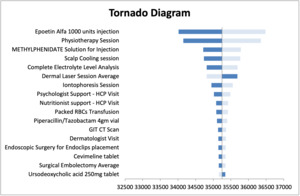
The sensitivity analysis results were presented in Supplementary Figure S2 and Supplementary Table S4. The tornado diagram presents the results of one-way sensitivity analysis (Figure 2). The unit cost of Epeotin alfa injection has the greatest impact on the results. The average probabilistic values resulted in population cost savings of SAR 11 005 210 (Intl$5 643 698) and per-patient cost savings of SAR 24 972 (Intl$12 805) for severe AEs, while resulting in average population cost savings of SAR 17 946 951 (Intl$9 203 564) and per-patient cost savings of SAR 39 231 (Intl$20 118) for all-grade AEs associated with treatment with CET + CT vs PAN + CT within the Saudi societal perspective model.
DISCUSSION
CRC is the third most common cancer occurring in humans worldwide and the second most common cancer in Saudi Arabia.4,21 Previously untreated patients with RAS wild-type mCRC may have received an EGFR inhibitor along with chemotherapy. The AEs profile for anti-EGFR, either the CET + CT regimen or the PAN + CT regimen, is highly influenced by their AE profiles.22
Based on the distributions reported in SmPCs,18,19 modeling of the frequency of AEs showed that patients treated with CET + CT had fewer AEs than those treated with PAN + CT. It is expected that a lower AEs frequency could result in lower AE management costs, elevated patient quality of life, and better therapy adherence with CET + CT vs PAN + CT within the Saudi healthcare setting.
Our economic model showed that treatment with CET + CT resulted in AE management cost savings for severe and all-grade AEs from the Saudi payer and societal perspectives compared with treatment with PAN + CT for patients with wild-type RAS mCRC in first-line settings within the Saudi healthcare system.
Similar economic studies that reported differences in AEs rates and AE management costs in first-line treatment with either CET or PAN regimens in patients with mCRC with wild-type RAS were conducted in different countries. In Italy, CET + CT resulted in fewer AEs; thus, markedly lower AE management costs were observed with CET + CT than with PAN + CT. This result suggests that CET + CT could decrease the cost burden on the Italian National Health Service and the AE burden for patients with mCRC.22 In Spain, lower rates of AEs for CET + CT have been reported in eligible patients with mCRC, which may result in a reduced AE burden, suggesting that treatment with CET + CT could yield annual AE management cost savings.23 In Latin American countries, such as Argentina, Brazil, and Panama, patients with mCRC and wild-type RAS treated with CET + CT are expected to experience fewer AEs than those treated with PAN + CT. Accordingly, this study recommends that lower frequency rates could result in lower overall and severe AEs’ management costs.24 Moreover, in Algeria, CET + CT was associated with a lower frequency of AEs than PAN + CT; thus, CET + CT resulted in lower AE management costs for patients with Algerian mCRC. The study concluded that the use of CET + CT might potentially alleviate the financial burden on the Algerian healthcare system.25
Our study had the following strengths. First, all model assumptions, inputs, and results were validated by Saudi physicians who specialize in treating mCRC and use both the CET + CT and PAN + CT regimens as treatment options. Second, the frequencies of common and very common AEs were sourced from the European SmPCs of CET and PAN, while the severity of these AEs was sourced from a published meta-analysis study. Third, we calculated indirect costs on patients to measure the economic burden of these AEs on society and the economy. Finally, a well-constructed sensitivity analysis was performed on the model input parameters based on the CHEERS recommendations26 to guarantee the robustness of the model.
Our economic analyses include the following limitations. First, the model used categorical data rather than numerical data on AEs, which is a simplification of the detailed information on AEs available in dossiers submitted to regulators. Second, within SmPCs, AEs are presented without distinguishing between treatment lines or indications. Third, experience with AE management and prevention over time can have an impact on the frequencies observed in healthcare institutions and thus on achievable savings. Fourth, the caregiver time and transportation costs were not considered in this study because there are no accurate local data about these costs, and there is great variability in the caregiver time and the transportation costs among the different patients’ socioeconomic levels. Therefore, we took a conservative approach to prevent any uncertainty. Fifth, the model lacks validation against real-world data to reflect the true costs and outcomes of AE management in practice, but there are no such local real-world data in KSA. We recommended collection of real-world data for AE severity in KSA in a separate research study. Finally, the number of mCRC patients with wild-type RAS eligible for anti-EGFR therapy was obtained from the relevant literature validated by our local Delphi panel owing to the unavailability of local official databases.
CONCLUSION
Considering that the regulatory labels for cetuximab and panitumumab was the basis for estimating the frequencies of adverse events, the CET + CT regimen was associated with a lower AEs frequency compared to the PAN + CT regimen for the treatment of Saudi untreated RAS wild-type mCRC patients, thus it could amount to AE management cost savings from both the Saudi payer and societal perspectives. These substantial cost savings may mitigate the financial burden of mCRC in Saudi healthcare settings.
Funding
This study was funded by Merck, Saudi Arabia, which had no involvement in the study design, analysis, interpretation of results, or manuscript writing. The funding received was used to pay for the submission and the open access publication fees.
Disclosures
G.E. was employed by HTA Office, LLC. G.E. is a speaker for Janssen, Merck, Novartis, AstraZeneca, Roche, Eva Pharma, and Pfizer. H.L. and M.O. were employed by Merck, Saudi Arabia. The authors have no other financial relationships to disclose.